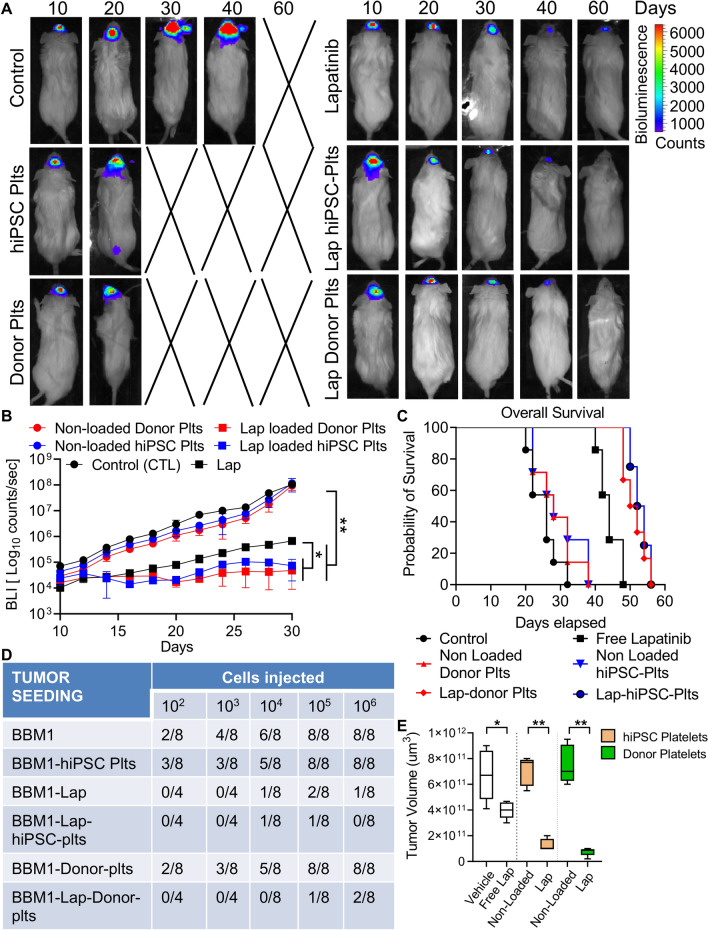
Figure 4.
Therapeutic efficacy of lapatinib-loaded hiPSC-platelets in vivo.(A) Representative BLI images of xenografted BBM1-derived tumor-bearing female NOD/SCID mice treated with vehicle (control), free lapatinib, and non-loaded and lapatinib-loaded hiPSC- and donor-derived platelets every 3 days. (B) Bioluminescence was quantified for BBM1-derived tumor-bearing female NOD/SCID mice throughout the experiment, as indicated. Mice were injected with vehicle (control), free lapatinib, and non-loaded and lapatinib-loaded hiPSC- and donor-derived platelets every 3 days. * indicates p < 0.05, ** indicates p < 0.001. Each group contains n = 7 mice. (C) Overall survival of variously treated BBM1-derived tumor-bearing female NOD/SCID mice. Control vs. non-loaded hiPSC- and donor-derived platelets, ns (non-significant); control vs. lapatinib, p < 0.05; control vs. lapatinib-loaded hiPSC- and donor-derived platelets, p < 0.001. (D) Tumor-seeding capability of BBM1 cells in female NOD/SCID mice pre-treated with vehicle (control), and non-loaded and lapatinib-loaded hiPSC and donor-derived platelets. (E) Tumor volume of BBM-derived tumor-bearing NOD/SCID mice treated with vehicle, free lapatinib, and non-loaded and lapatinib-loaded hiPSC- and donor-derived platelets, measured at 40 days post-implant (n = 7 per group). Control vs. lapatinib, p < 0.05; control vs. free lapatinib, * p < 0.05; non-loaded platelets vs. lapatinib-loaded platelets, ** p < 0.001.