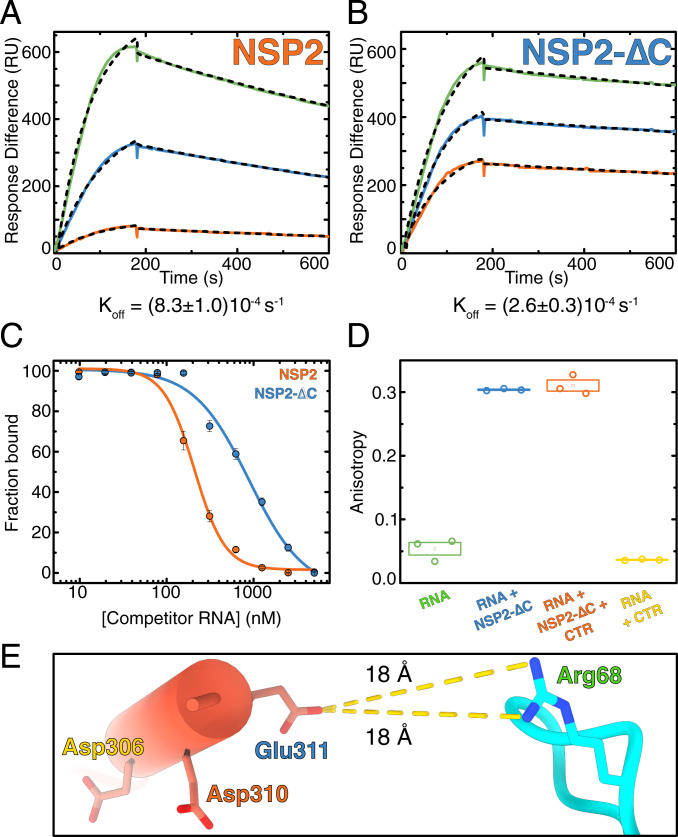
Fig. 5.
The CTR promotes RNA dissociation noncompetitively. (A and B) SPR sensograms of NSP2 (A) and NSP2-∆C (B) binding to RNA. Although NSP2-∆C binds RNA with an approximately sixfold higher affinity, this is due to a modest (1.5-fold) increase in Kon and a larger (3.2-fold) decrease in Koff. (C) RNA competition assay. The fractional binding of fluorescently labeled RNA was determined by fluorescence anisotropy. Labeled RNA (10 nM) fully bound to NSP2 (orange) or NSP2-∆C (blue) was titrated with unlabeled RNA of identical sequence to compete for NSP2 binding against labeled RNA. The IC50 values for NSP2 and NSP2-∆C are 208 ± 11 nM and 890 ± 160 nM, respectively. The NSP2–RNA complex undergoes strand exchange more readily than the NSP2–∆C:RNA complex. (D) RNA binding by NSP2 in the presence of the CTR peptide. CTR peptide (10 µM) was added to preformed NSP2–∆C:RNA complexes. The CTR peptide does not compete with RNA for binding to NSP2-∆C. (E) Estimated distances between acidic residues within the CTR and the R68 that interacts with RNA. Note the nearest side chain (E311), which is 18 Å away from R68.