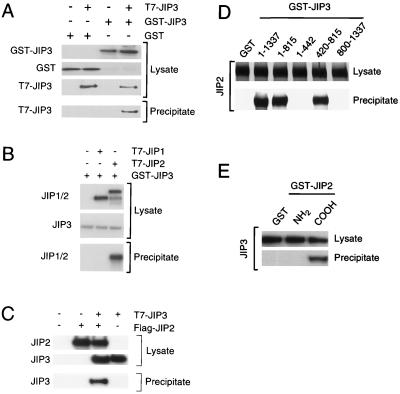
FIG. 8.
JIP3 selectively interacts with JIP scaffold proteins. (A) Epitope (T7)-tagged JIP3a was coexpressed with GST-tagged JIP3a in COS7 cells and incubated with glutathione-agarose. Bound proteins were detected by immunoblot analysis with an antibody to the T7 epitope. Expression of T7-JIP3 and GST-JIP3 proteins in the cell lysates was examined by immunoblot analysis. (B) Epitope (T7)-tagged JIP1 and JIP2 were expressed in COS7 cells. JIP3a was also expressed as a GST fusion protein in COS7 cells and immobilized on glutathione-agarose beads. Bound proteins were detected by immunoblot analysis with an antibody to the T7 epitope. Expression of T7-JIP1, T7-JIP2, and GST-JIP3a proteins in the cell lysates was monitored by immunoblot analysis. (C) Epitope-tagged T7-JIP3a and Flag-JIP2 were expressed in COS-7 cells. Lysates were prepared, and the amount of JIP3 and JIP2 was examined by immunoblot analysis using monoclonal antibodies to the T7 and Flag epitopes. The Flag-JIP2 was immunoprecipitated with antibody M2, and T7-JIP3 in the immunoprecipitates was detected by immunoblot analysis with an antibody to the T7 epitope tag. (D) Deletion analysis of JIP3. To define the JIP2 binding region of JIP3a, fragments of JIP3a (residues 1 to 1337, 1 to 815, 1 to 442, 420 to 815, and 800 to 1337) fused to GST were immobilized on glutathione-agarose. Control experiments were performed with immobilized GST. These immobilized proteins were incubated with JIP2 prepared by in vitro translation in the presence of [35S]methionine. Binding of JIP2 to the immobilized proteins was examined following SDS-PAGE by autoradiography. (E) Deletion analysis of JIP2. To define the JIP3 binding region of JIP2, fragments of JIP2 fused to GST were immobilized on glutathione-agarose. Control experiments were performed with GST. These immobilized proteins were incubated with JIP3a prepared by in vitro translation in the presence of [35S]methionine. The binding of JIP3 to an NH2-terminal fragment of JIP2 (residues 1 to 229) and a COOH-terminal fragment of JIP2 (residues 557 to 824) was examined following SDS-PAGE by autoradiography.