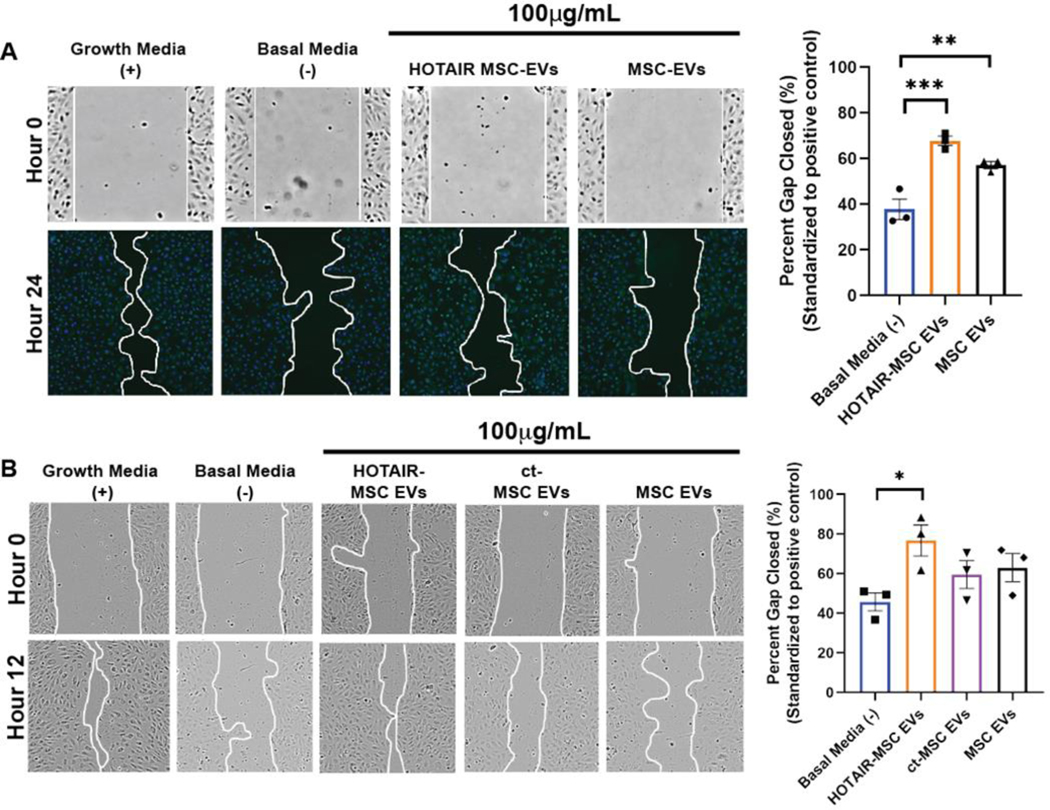
Figure 3. In vitro assessment of HOTAIR-MSC EV activity.
(A) HDMECs were treated with growth media only (EGM; positive control), basal media only (EBM; negative control), 100 μg/mL HOTAIR-MSC EVs in basal media, or 100 μg/mL native MSC EVs in basal media. Images were taken at time 0 (brightfield) and 24h post wounding of the cell layer (DAPI-stained). Representative images for each group and timepoint are displayed and the gap area outlined with a white line. (B) HUVECs were treated with growth media (EGM; positive control), basal media (EBM; negative control), 100 μg/mL HOTAIR-MSC EVs in basal media, 100 μg/mL ct-MSC EVs in basal media, or 100 μg/mL native MSC EVs in basal media. Brightfield images were taken at time 0 (brightfield) and 12h post wounding of the cell layer. Representative images for each group and timepoint are displayed and the gap area outlined with a white line. For both A and B, data are compared to growth media positive control values (set to 100%) and statistical significance was calculated using one-way ANOVA with Tukey’s test (* p<0.05, **p<0.01, ***p<0.001) (n=3).