Figure 2. Structural components of titin unfold at different extension forces, imparting a wide range of extensibility.
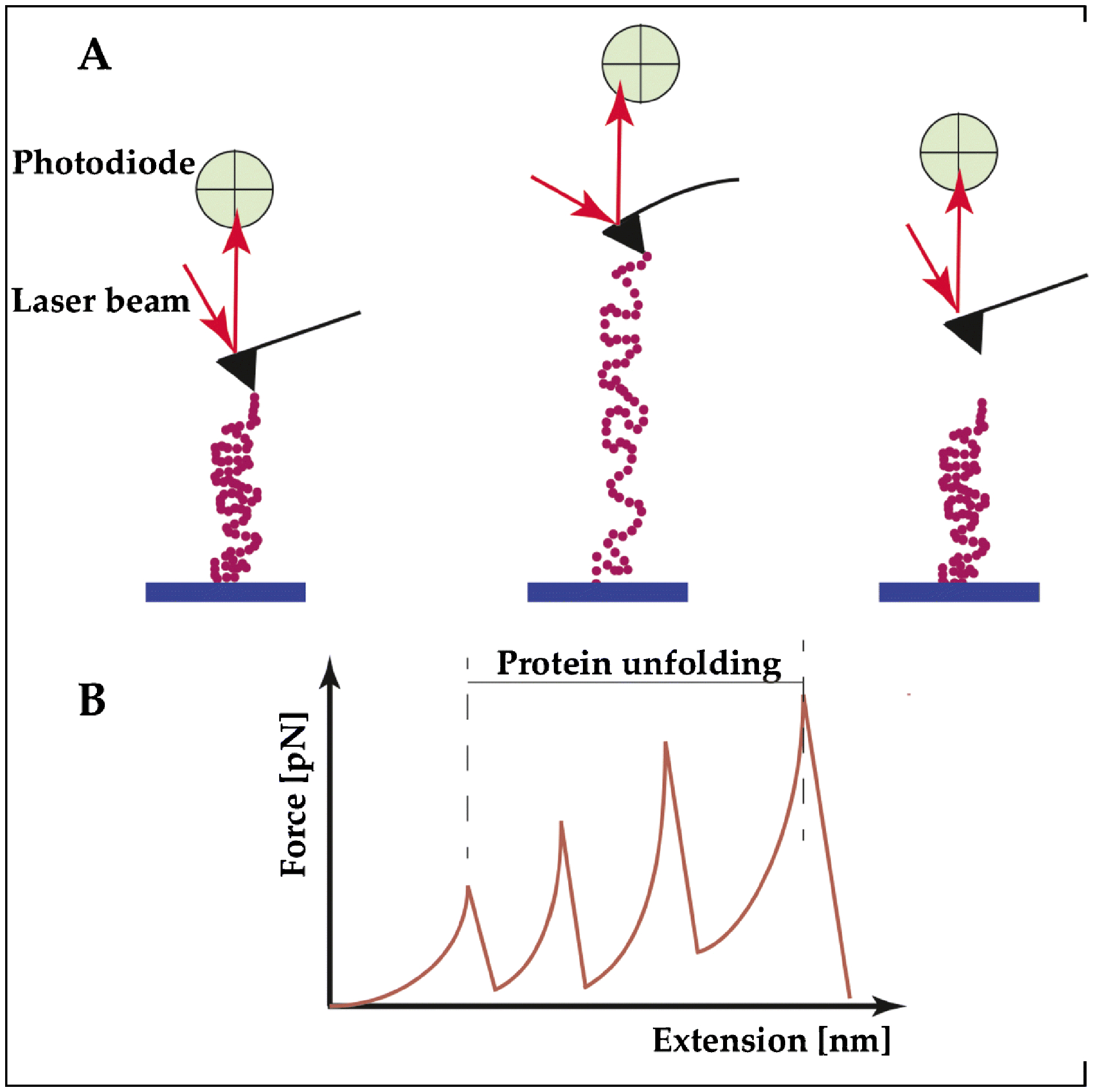
(A) Example of a single-molecule force spectroscopy using the atomic force microscope (AFM) to study the contribution of the individual building blocks of titin under stretching. Left: The TTN segment to be tested is deposited on a substrate, and then attached at one end at the tip of an elastic AFM cantilever. Middle: The peptide is pulled up by a piezoelectric controller. When the proteins start to unfold, the AFM cantilever is deflected and its deflection is measured by a laser beam. This deflection is transformed in a force curve. Right: The TTN peptide detaches when it reaches the maximum stretch. (B) Titin proteins have an intrinsic elasticity that causes them to remain rigid at small length scales yet display significant flexibility over longer lengths. One of the theories that describes this behavior and the progressive unfolding of the different segments of TTN is the “worm-like chain” model of semiflexible polymers, as shown in panel B.
