Abstract
Background
The Coronavirus disease 2019 (COVID-19) pandemic has led to the rapid development of COVID-19 vaccine. The Centers for Disease Control and Prevention (CDC) has recently reported increase in myopericarditis incidence post-COVID-19 vaccination. Post-vaccination myopericarditis as side effect has been reported, however, is infrequent. We described a case of pericarditis post-first dose of Pfizer-BioNTech vaccine.
Case summary
A patient presented with typical symptoms of pericarditis and related electrocardiogram and echocardiogram changes, 7 days post receiving the first dose of COVID-19 vaccine. No other causes were identified from series of investigations. Patient had good symptomatic relief with non-steroidal anti-inflammatory medication.
Discussion
The incidence of pericarditis post-vaccination is rare, with limited reporting in previous literatures. No causal relationship has yet to be established due to small number of cases. The benefits of COVID-19 vaccination currently outweigh the side effect profile and are recommended as the first-line approach to control the current pandemic.
Keywords: Case report, COVID-19, Vaccine, Myopericarditis, Pericarditis
Learning points
Inflammatory heart disease, including myocarditis and pericarditis, is an acknowledged side effect of mRNA type of COVID-19 vaccine.
Majority cases of myopericarditis secondary to vaccination responded well with short course of anti-inflammatory therapy and minimal intervention.
The Centers for Disease Control and Prevention (CDC) continues to recommend COVID-19 vaccine as myopericarditis incidence post-vaccination remains to be low and the benefits currently outweigh the risk; however, the decision should be patient-specific and includes multi-disciplinary discussion.
Introduction
The novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection was first identified in December 2019 and has since been declared as a global pandemic by the World Health Organisation (WHO).1 The overwhelming burden of Coronavirus disease 2019 or better known as COVID-19 has led to rapid development of the COVID-19 vaccine. The Pfizer-BioNTech and Moderna vaccines subtype were developed on the basis of modified messenger RNA that encodes the SARS-CoV-2 ‘spike proteins’, an important protein located on the outer surface of the virus.2
The common side effects of the COVID-19 vaccine include fatigue, myalgia, headache, and local reaction. More serious side effects, such as severe allergic reaction, are seen in ∼1 in 100 000 people. However, as these vaccines are relatively new, the documented long-term side effects profile were limited.3 Inflammatory cardiac disease, including pericarditis and myocarditis post-vaccination, has been reported in multiple literatures; however, it remained as rare side effects of vaccination.
The Centers for Disease Control and Prevention (CDC) has recently reported the increase in cases of myocarditis and pericarditis after mRNA subtype of COVID-19 vaccination since April 2021 in the USA. Cases reported through the Vaccine Adverse Event Reporting System (VAERS) were mostly in male adolescents and young adults aged 16 years or older, and more often within several days after the second dose of COVID-19 vaccine.4
In this report, we described a case of pericarditis following the first dose of Pfizer-BioNTech COVID-19 vaccine.
Timeline
| Date | Event |
|---|---|
| 7 days prior to admission |
|
| Day 1 admission |
|
| Day 2 admission |
|
| Day 3 admission |
|
| 7 weeks post-admission |
|
Case presentation
A 66-year-old Caucasian man presented to the hospital with positional sharp chest pain at rest, which was worse on lying flat and was associated with shortness of breath and cough. Medical history included type 2 diabetes mellitus, depression, and previous transient ischaemic attack. He had no documented drug allergy or previous allergic reaction. He received the first dose of Pfizer-BioNTech vaccine 7 days prior to developing the described symptoms.
On admission, he was tachycardic and hypotensive, with heart rate 116 and blood pressure 85/60. The rest of vital signs were within the normal range—respiratory rate 16, oxygen saturation 97% on room air, and he was afebrile. Clinical examination was unremarkable with normal heart sounds, non-raised jugular venous pressure, and no pericardial rub were heard on auscultation. Chest X-ray was normal. Electrocardiogram (ECG) showed tachycardia, with widespread concave ST elevation in most leads (lead I, II, AVL, AVF, and V4–V6) and the Spodick’s sign—down-slopping TP segment best-visualized in lead II and V2–V6 (Figure 1). The laboratory investigations were as follows—borderline leukocytosis and neutrophilia—white cell count of 10.4 × 109/L (4–10 × 109/L) and neutrophils of 8.08 × 109/L (2–7 × 109/L), elevated C-reactive protein of 94 mg/L (0–5 mg/L), urea 9.8 mmol/L (2.5–7.8 mmol/L), and creatinine 107 µmol/L (62–106 µmol/L). COVID-19 swab was negative. Blood culture showed no significant growth. First serum troponin was negative <0.01 and the second level 3 h later was slightly raised 0.08 µg/L (<0.04 µg/L). Atypical and serology screen including viral hepatitis, Cytomegalovirus (CMV), Human immunodeficiency virus (HIV), and Aspergillus were unremarkable except for previous past Epstein-Barr virus (EBV) infection. QuantiFERON and Autoimmune screen were negative.
Figure 1.
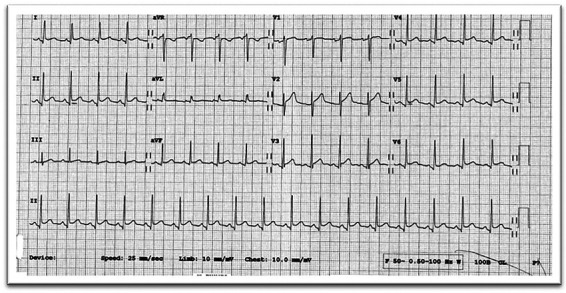
Electrocardiogram on admission—concave ST elevation on lead I, II, AVL, AVF, V4–V6, and down-slopping TP segment in lead II and V2–V6 (Spodick’s sign).
Echocardiogram showed pericardial fluid around entire heart measuring up to 1.5 cm with no evidence of cardiac tamponade, and good left and right ventricular function with mild basal septal hypertrophy (Figure 2). Patient was treated with paracetamol and ibuprofen three times daily as inpatient, and showed clinical improvement after 2 days. Patient was discharged home after 3 days of hospital admission. Repeat echocardiogram a month later showed resolution of pericardial fluid (Figure 3), and repeat ECG showed normal sinus rhythm (Figure 4). Patient’s case was discussed with an immunologist, who advised the patient not to receive the second dose of the vaccine, due to his possible susceptibility to immune-related vaccine reaction.
Figure 2.

Echocardiogram on admission—pericardial effusion around the heart.
Figure 3.
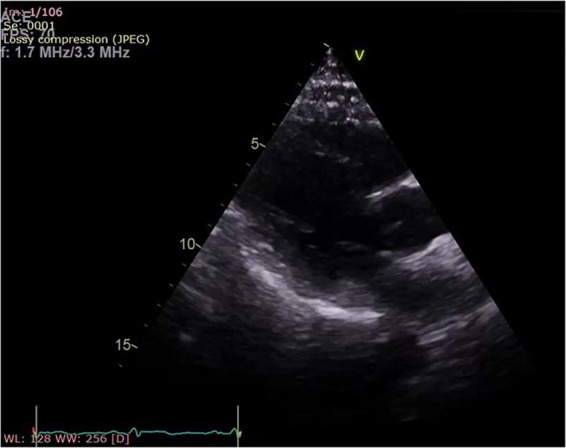
Echocardiogram on 7-week follow-up—resolution of pericardial effusion.
Figure 4.
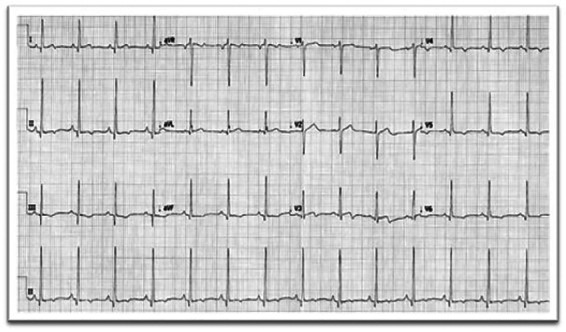
Electrocardiogram on 7-week follow-up—normal sinus rhythm with resolution of ST elevation.
Discussion
Pericarditis is defined as the inflammation of pericardial sac and is recorded in approximately five percent of patients presented to the emergency department with non-ischaemic chest pain.5 The diagnosis is established based on at least two of the following criteria: (i) chest pain typically characterizes as sharp and positional, (ii) pericardial friction rub, (iii) characteristic ECG changes (widespread ST depression), or (iv) pericardial effusion. More definitive diagnosis could be achieved by cardiac magnetic resonance or pericardiocentesis and pericardial biopsy; however, such studies are rarely done in majority of patients with uncomplicated acute pericarditis.
The aetiology of acute pericarditis can be generally classified as infectious and non-infectious causes, and largely dependent on epidemiological background, patient population, and clinical setting. Approximately up to 50% of cases in developed countries are diagnosed as idiopathic or post-viral cause.6
The overwhelming burden of SARS-CoV-2 infection has led to rapid development of COVID-19 vaccines. A study published in the New England Journal of Medicine (NEJM) has showed that two-dose regimen of BNT162b2 (Pfizer-BioNTech) has 95% efficacy for protection against COVID-19 in the persons 16 years of age or older.7 The Centers for Disease Control and Prevention has recently reported the increase in cases of myocarditis and pericarditis since April 2021 in the USA.4 Up to 31 May 2021, a total of 528 cases has been reported through VAERS after receiving both doses of the mRNA-based COVID vaccine, with calculated incidence rate of 8.6% per million doses in 21-day risk interval.8
A paediatric case series reported acute myopericarditis in seven healthy male adolescents aged 14–19 years old, within 4 days after receiving the second dose of Pfizer-BioNTech vaccine. All seven patients had rapid resolution of their symptoms, with three of them treated with non-steroidal anti inflammatory drugs (NSAIDs) only and four of them received intravenous immune globulin (IVIG) and corticosteroids.9
Another case report described a case of 62-year-old man with history of familial Mediterranean fever (FMF) treated with prophylactic colchicine. He presented 8 days after receiving the first COVID-19 vaccine—vaccine type not disclosed in the report. In this case, patient was unstable on admission requiring urgent pericardiocentesis. The authors were able to exclude FMF as the cause of pericarditis.10
As vaccination programme for COVID-19 is still in the early phase, data on the pericarditis incidence secondary to COVID-19 vaccine in the literatures are limited. Myopericarditis after any type of vaccination has also been reported in multiple literatures mainly in series of case reports; however, the incidence remains to be extremely rare with no definite causal relationship yet to be established. The incidence rate of myopericarditis following vaccination is estimated to be 0.24 per 100 000 vaccinees, which is similar when compared with background incidence rate of myopericarditis of 0.95 per 100 000 population.11 The hypothetical pathophysiological mechanism of vaccination-related pericarditis is based on the immunologic systemic reactivity to the vaccine’s contents.12
A study has reported two cases of symptomatic pericarditis after Influenza vaccination. The two cases of pericarditis presented after 5 and 6 days of post-vaccination, respectively, with other causes excluded from series of investigations. In both cases, the disease has resolved with only oral anti-inflammatory medication and no complication was reported.13
Another case report described a male patient with two consecutive diagnosis of pericarditis 1 year apart, and in both presentations, influenza vaccination was administered a few days prior to onset of symptoms. The patient was then monitored for subsequent 6 years during which the influenza vaccination was not received, and no recurrence of pericarditis was reported.14 This study described a positive rechallenge (second presentation of pericarditis after influenza vaccination) and positive de-challenge (no presentation of pericarditis after not receiving influenza vaccine), and suggested a possible causal relationship between influenza vaccine and pericarditis.
A retrospective study looked into 84 cases of confirmed diagnosis of pericarditis, and divided them into two different groups—Group A with a known cause of pericarditis, and Group B that was diagnosed with idiopathic pericarditis. Interestingly, none of patients in Group A received previous influenza vaccination, while all Group B patients received influenza vaccination prior to symptoms onset. The study also showed that the group labelled as ’idiopathic’ pericarditis had a seasonal distribution of pericarditis that coincided with period of influenza vaccination.15
Majority of cases of myopericarditis post-vaccination discussed above responded well to a short course of anti-inflammatory medication with no complication reported on follow-up, which is similar to our case. However, one case reported large pericardial effusion requiring urgent pericardiocentesis and another case series described four paediatric cases requiring adjunct therapy with IVIG and corticosteroids. In our case, patient’s symptoms were well-controlled with NSAIDs therapy alone, which aligned with the European Society of Cardiology (ESC)’s recommendation for first-line therapy of acute pericarditis. In a more complicated symptoms or delayed resolution, dual therapy with colchicine or corticosteroid may be beneficial. Recent advances have also introduced novel pharmacotherapies, such as anti-interleukin-1 for refractory idiopathic recurrent pericarditis.16
Conclusions
Based on the interval report produced by CDC, the incidence of myopericarditis post-COVID-19 vaccination pericarditis remains to be low similar to incidence of myopericarditis post-vaccination of any type. The majority of cases has a short course of disease and requiring minimal medical intervention. CDC continues to recommend COVID-19 vaccination and advocate for health practitioners to continue reporting all cases of myopericarditis post-COVID-19 vaccination to VAERS. SARS-CoV-2 infection is highly contagious, and COVID-19 can lead to severe respiratory syndrome leading to hospitalization and increased mortality and morbidity risk. The demonstrated benefits of COVID-19 vaccine far outweigh the possible risks, and currently, the most promising approach to curb the COVID-19 pandemic.
Lead author biography

Sarah Ashaari is currently a Non-Consultant Hospital Doctor (NCHD) in general medicine in South Hospital Group, Health Service Executive (HSE), Ireland. She graduated from University College Cork with special interest in Cardiology and Respiratory Medicine.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors thank everyone who was involved directly and indirectly in the data collection and writing process of this case report.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
References
- 1.World Health Organisation. WHO Director-General's Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 (15 February 2021).
- 2.Centers of Disease Control and Prevention. Understanding and Explaining mRNA COVID-19 Vaccines. CDC. 2020. https://www.cdc.gov/vaccines/covid-19/hcp/mrna-vaccine-basics.html. (14 February 2021).
- 3.Health Service Executive. Side Effects of COVID-19 Vaccines. 2021. https://www2.hse.ie/screening-and-vaccinations/covid-19-vaccine/side-effects-covid-19-vaccine.html (14 February 2021).
- 4.Centers of Disease Control and Prevention. Clinical Considerations: Myocarditis After mRNA COVID-19 Vaccines. CDC. 2021. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html (19 June 2021).
- 5.Skoularigis J, Xanthopoulos A.. Diagnosis of Acute Pericarditis. 2017. Escardio.org. https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-15/Diagnosis-of-acute-pericarditis (15 February 2021).
- 6.Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J. et al. ; ESC Scientific Document Group. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur Heart J 2015;36:2921–2964.26320112 [Google Scholar]
- 7.Polack F, Thomas S, Kitchin N, Absalon J, Gurtman A, Lockhart S. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimabukuro T. Covid-19 Vaccine Safety Updates. fda.gov. 2021. https://www.fda.gov/media/150054/download (19 June 2021).
- 9.Marshall M, Ferguson I, Lewis P, Jaggi P, Gagliardo C, Collins J. et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics 2021;148:e2021052478. [DOI] [PubMed] [Google Scholar]
- 10.Rozin AP, Yalonetsky S.. Images of COVID-19 vaccination. Int J Case Reports Clin Images 2021;3:144–146. [Google Scholar]
- 11.Kuntz J, Crane B, Weinmann S, Naleway A.. Myocarditis and pericarditis are rare following live viral vaccinations in adults. Vaccine 2018;36:1524–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramasamy V, Mayosi B, Sturrock E, Ntsekhe M.. Established and novel pathophysiological mechanisms of pericardial injury and constrictive pericarditis. World J Cardiol 2018;10:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Meester A, Luwaert R, Chaudron J.. Symptomatic pericarditis after influenza vaccination. Chest 2000;117:1803–1805. [DOI] [PubMed] [Google Scholar]
- 14.Mei R, Raschi E, Poluzzi E, Diemberger I, De Ponti F.. Recurrence of pericarditis after influenza vaccination: a case report and review of the literature. BMC Pharmacol Toxicol 2018;19: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanettini MT, Zanettini JO, Zanettini JP.. Pericarditis. Series of 84 consecutive cases. Arq Bras Cardiol 2004;82:360–369. [English, Portuguese]. [DOI] [PubMed] [Google Scholar]
- 16.Tombetti E, Mulè A, Tamanini S, Matteucci L, Negro E, Brucato A. et al. Novel pharmacotherapies for recurrent pericarditis: current options in 2020. Curr Cardiol Rep 2020;22:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


