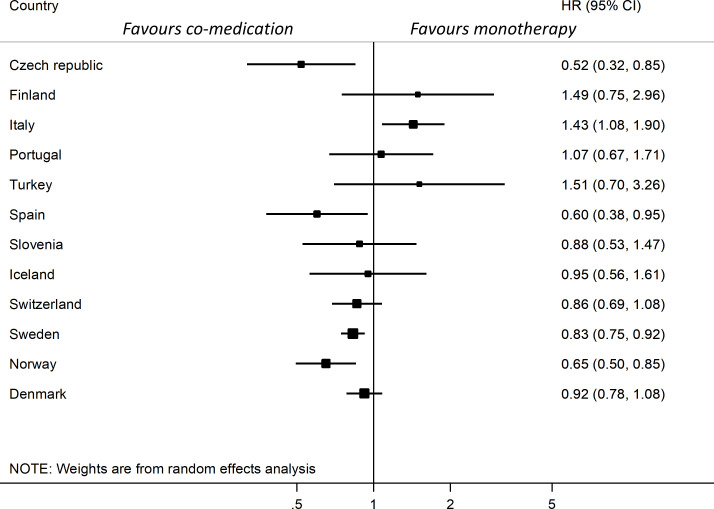
Figure 2.
Forest plot of country-specific hazard ratios for TNFi discontinuation at 12 months comparing TNFi and csDMARD co-medication with TNFi monotherapy, ordered by overall TNFi retention rate per country. Adjusted for baseline age, sex, calendar year, DAS28 and disease duration. Combined results are not presented due to significant heterogeneity. Data from Romania are not presented due to <5 patients with monotherapy. csDMARDs, conventional synthetic disease-modifying anti-rheumatic drugs; DAS28, Disease Activity Score with 28 joints; TNFi, tumour necrosis factor inhibitors.