Figure 2 .
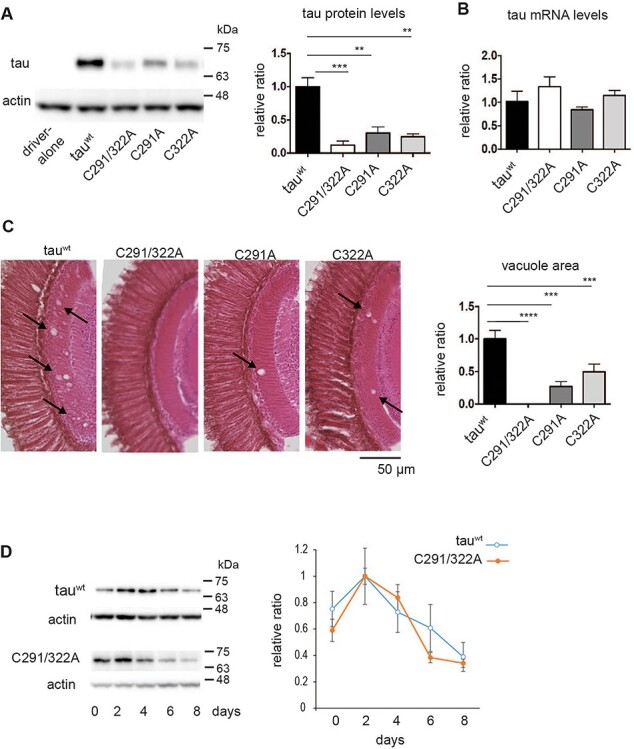
Cysteine residues stabilize tau proteins in the fly retina. (A) Tau protein levels analyzed by western blotting of head extracts with anti-tau antibody (T46). Actin was used as a loading control (means ± SE, n = 4, *P < 0.05, Student’s t-test). (B) mRNA levels analyzed by RT-qPCR. No significant differences were detected (mean ± SD, n = 3, one-way ANOVA followed by Tukey’s HSD multiple comparisons test). Flies were 2 days old after eclosion. (C) Substitution of cysteine residues to alanine mitigates tau toxicity in a Drosophila model. Neurodegeneration in the lamina caused by tau was observed as an increase in the vacuole area (indicated by arrows). Quantitation of the vacuole area is shown on the right (mean ± SE, n.s., n = 3, ****P < 0.0001, ***P < 0.001, **P < 0.01, one-way ANOVA followed by Tukey’s HSD multiple comparisons test). Flies were 10 days old. (D) Turnover of tauC291/322A is faster than that of tauwt in the fly retina. Tau expression was driven by elav-GeneSwitch. Newly eclosed flies were fed RU486-containing food for 2 days, then transferred to food without RU486 (day 0). Flies were collected every 2 days, and head homogenates were subjected to western blotting with anti-tau antibody (T46). TauC291/322A levels were significantly lower at day 6 or at day 8 compared to day 2, while tauwt levels were not (P < 0.001 for tauC291/322A, one-way ANOVA with post hoc Tukey HSD). Mean ± SE, n = 3.
