Figure 4 .
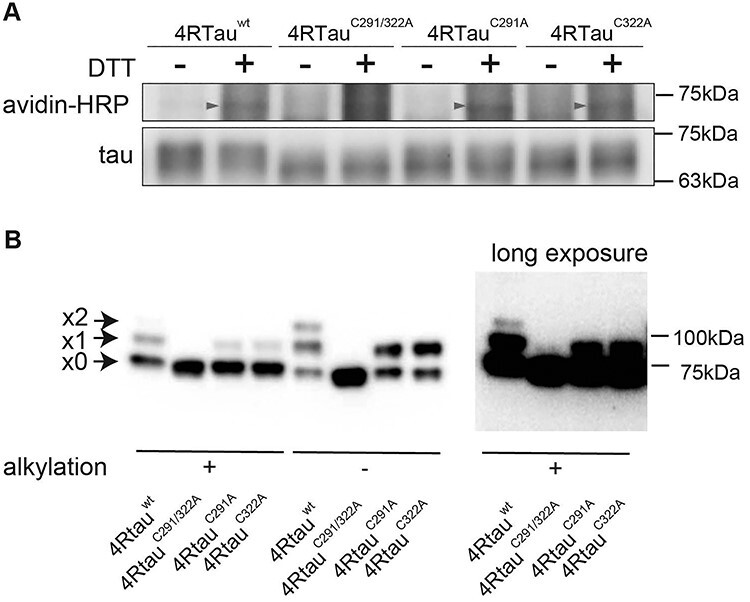
Tau forms disulfide bonds in mouse primary cultured neurons and the fly retina. (A) 4Rtauwt, 4RtauC291/322A, 4RtauC291A and 4RtauC322A were expressed in mouse primary cortical neurons and subjected to BIAM analyses. Biotinylation was observed with tauwt, tauC291A and tauC322A, but not with tauC291/322A (avidin-HRP, arrowheads). The 2N4R isoform of tau was used. (B) Tau forms disulfide bonds via C291 or C322 in the fly retina. Fly head lysates were first treated with an alkylating reagent to block free thiols (alkylation [+]). Lysates were then treated with DTT to reduce disulfide bonds and incubated with m-PEG to label the reduced cysteine residues. Tau forming disulfide bonds were detected by PEG-maleimide labeling in fly heads expressing 4Rtauwt, 4RtauC291A or 4RtauC322A, but not those expressing 4RtauC291/322A. Samples were also prepared without alkylation, which exposes all cysteines for tagging if there is no oxidation (alkylation [−]). Arrowheads from the top indicate two, one or zero cysteine residues forming disulfide bonds. The top two bands were not observed with 4RtauC291/322A even after long exposure (right, long exposure).
