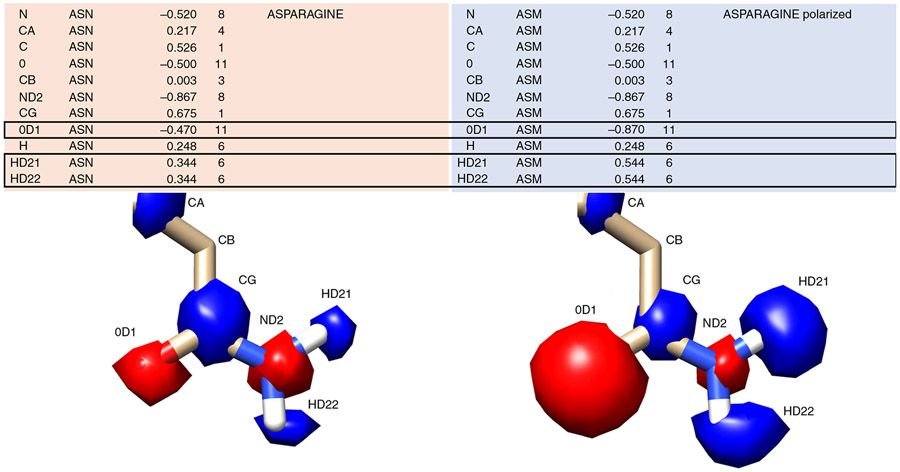
Fig. 8 ∣. Polarizing effects specific atoms’ electrostatic potential.
In contrast to global modifications to the electrostatic potential with the incorporation of thin spheres, polarizing allows for very specific modifications to a residue’s charge status. A canonical asparagine (ASN) from the prot.table.ambcrg.ambH file is shown with its polarized version ASM in which the carbonyl becomes more electronegative while the amide hydrogens become more electropositive to maintain the overall charge. The electrostatic potential corresponding to each atom is shown as spheres, with red corresponding to negative charge and blue corresponding to positive charge.