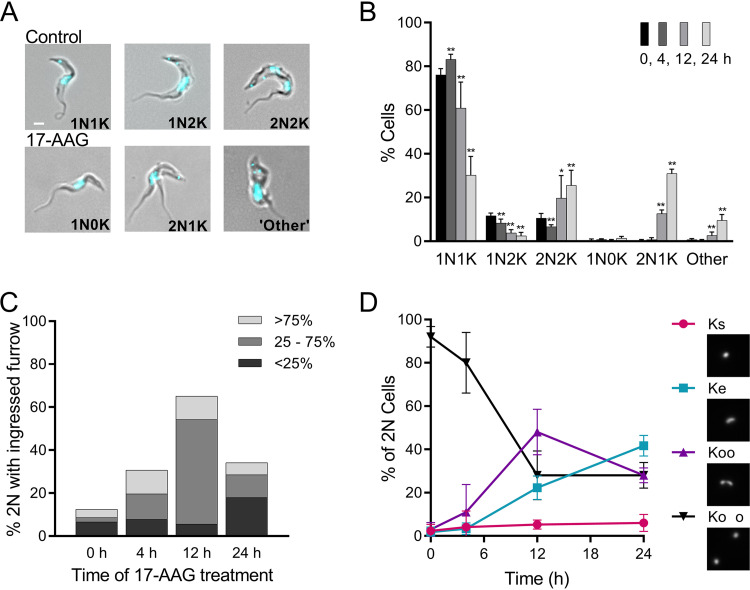
FIG 2.
Effects of increasing exposure to 17-AAG on kinetoplast segregation and cleavage furrow ingression. (A) Merged phase-contrast and DAPI fluorescence images of untreated controls and trypanosomes treated with 100 nM 17-AAG. In controls, the cell cycle progresses from 1 nucleus (N) and 1 kinetoplast (K) (1N1K) to 1 nucleus and 2 kinetoplasts (1N2K), followed by 2 nuclei and 2 kinetoplasts (2N2K) before cell division (Fig. 1). With 17-AAG treatment, abnormal nuclear and kinetoplast configurations occur, including 1N0K, 2N1K, or multiple nuclei and/or kinetoplasts (other). White bar, 1 μm. (B) Nuclear and kinetoplast content of trypanosomes over time with 100 nM 17-AAG treatment. Data are mean ± SD of ≥3 independent experiments; n = 3 to 11 at each point. *, P < 0.05; **, P < 0.01; t test, two-tailed equal variance to 0-h control. (C) Impact of 100 nM 17-AAG on cleavage furrow ingression. Cells were sampled at indicated times after start of 17-AAG treatment and examined for 2N cells with visible cleavage furrows (>50 cells scored per time point). Populations were subdivided by extent of furrow ingression. (D) Effect of 100 nM 17-AAG on kinetoplast separation. 2N cells in timed samples stained with DAPI were examined by fluorescence microscopy and scored as having the expected two fully separated kinetoplasts (Ko o, black; Fig. 1f), or as being abnormal, including those with directly adjacent kinetoplasts (Koo, purple), a single elongated kinetoplast (Ke, blue), or a single kinetoplast (Ks, red). Insets, example images of network morphologies. Mean ± SD of ≥3 independent experiments; >175 2N cells for each time point.