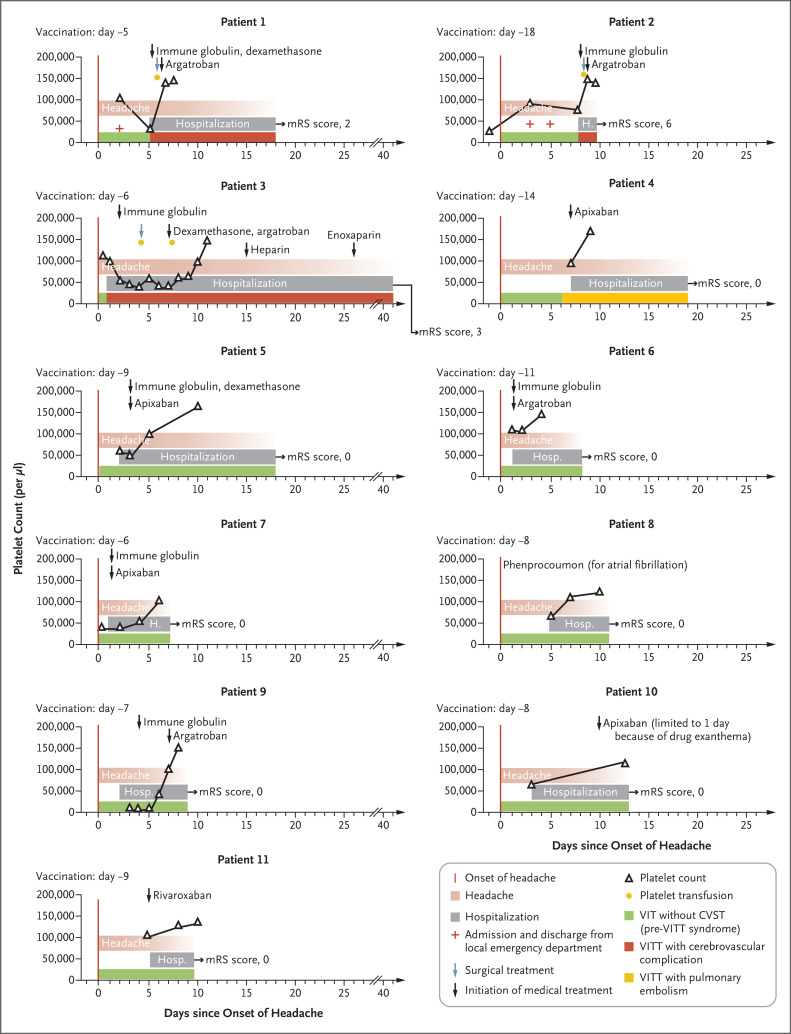
Figure 1. Clinical and Laboratory Data for Patients with VIT and Severe Headache (Pre-VITT Syndrome).
Shown are the time courses of the manifestation of pre-VITT syndrome (defined by headache onset), hospital admission (including emergency department admission and discharge in Patients 1 and 2), platelet counts, and cerebrovascular complications (in Patients 1, 2, and 3), as well as medical and neurosurgical treatment (decompressive craniectomy). In each graph, the number of days since the onset of headache is shown on the x axis and platelet counts on the y axis. Outcomes were assessed with the modified Rankin scale (mRS); scores on the scale range from 0 to 6, with higher scores indicating greater disability (0 indicates no symptoms, and 6 indicates death). CVST denotes cerebral venous sinus thrombosis, VIT vaccine-induced thrombocytopenia, and VITT vaccine-induced immune thrombotic thrombocytopenia.