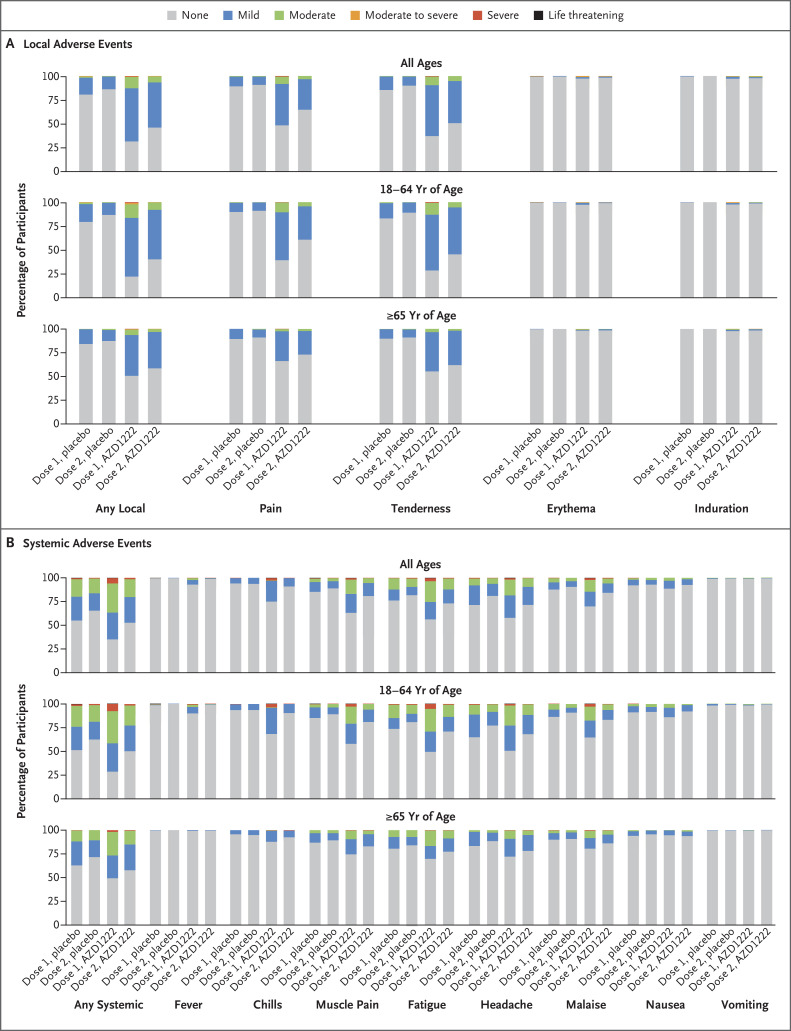
Figure 2. Local and Systemic Solicited Adverse Events after First and Second Dose, by Age Group.
Erythema and induration were classified by size as mild (2.5 to 5 cm), moderate (5.1 to 6 cm), or moderate-to-severe (>6 cm). Fevers were graded by temperature as none (≤37.8°C), mild (37.9 to 38.4°C), moderate (38.5 to 38.9°C), severe (39.0 to 40.0°C), or life threatening (≥40.1°C). The most common solicited adverse events that occurred in at least 5% of participants within 7 days after any dose in either group were tenderness (68.4% in the AZD1222 group and 19.0% in the placebo group) and pain (58.3% and 15.7%), both local adverse events; the most common systemic adverse events were headache (50.2% in the AZD1222 group and 35.5% in the placebo group), fatigue (49.7% and 31.2%), muscle pain (41.9% and 19.5%), malaise (35.0% and 17.0%), chills (28.2% and 9.5%), nausea (15.3% and 12.1%), and temperature higher than 37.8°C (7.0% and 0.6%). The All Ages group included 1013 participants for dose 1, placebo; 968 for dose 2, placebo; 2037 for dose 1, AZD1222; and 1962 for dose 2, AZD1222. The age 18 to 64 group included 663 participants for dose 1, placebo; 629 for dose 2, placebo; 1339 for dose 1, AZD1222; and 1288 for dose 2, AZD1222. The age 65 and older group included 350 participants for dose 1, placebo; 339 for dose 2, placebo; 698 for dose 1, AZD1222; and 674 for dose 2, AZD1222.