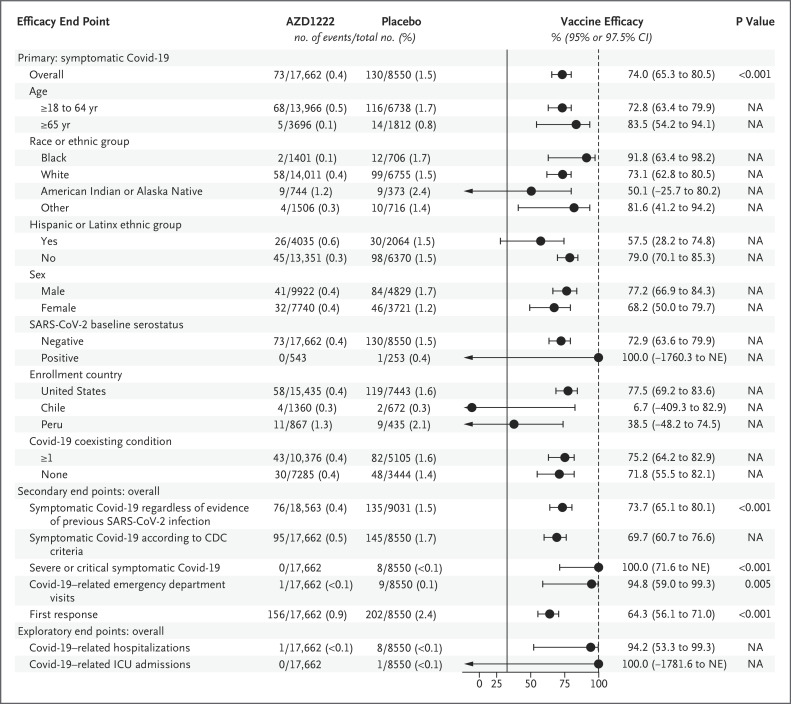
Figure 3. Estimated Vaccine Efficacy ≥15 Days after the Second Dose (Fully Vaccinated Analysis Population).
Values shown for no. of events/total no. are the number of events that occurred among the participants within each group and do not account for censoring due to unblinding of group assignment or loss to follow-up. The primary efficacy end point is the first case of SARS-CoV-2 RT-PCR–positive symptomatic illness occurring 15 days or more after the second dose of AZD1222 or placebo among participants with negative serostatus at baseline. Vaccine efficacy is shown with 95% confidence intervals (CIs), except for vaccine efficacy values for the primary efficacy end point according to SARS-CoV-2 baseline serostatus, the secondary end point of severe or critical symptomatic Covid-19, and the exploratory end point of Covid-19–related intensive care unit (ICU) admissions, which are based on a one-sided 97.5% CI calculated with the exact Poisson model, owing to nonconvergence of the Poisson regression with robust variance. Race and ethnic group were reported by the participant. Other denotes participants who provided a race or ethnic group identification other than White, Black, or American Indian or Alaska Native. Key secondary end points were incidence of symptomatic illness (at 15 days or more after the second dose of AZD1222 or placebo) regardless of evidence of previous SARS-CoV-2 infection at baseline, severe or critical symptomatic Covid-19 (at 15 days or more after the second dose of AZD1222 or placebo), Covid-19–related emergency department visits, symptomatic Covid-19 as defined by Centers for Disease Control and Prevention (CDC) criteria, and first response (change from negative serostatus for SARS-CoV-2 nucleocapsid antibodies at baseline to positive serostatus after receiving AZD1222 or placebo). P values are reported for the primary and key secondary outcomes; analyses followed prespecified plan to adjust for multiple comparisons. I bars indicate confidence intervals; arrows indicate truncated values, with actual values shown in the accompanying column; the dashed vertical line represents the upper limit (i.e., 100% vaccine efficacy); and the solid vertical line represents the nominally statistically significant criterion of a lower confidence interval greater than 30% applicable to the primary end point and is shown for reference. NA denotes not available, and NE could not be estimated.