Abstract
More than a year after the start of the COVID-19 pandemic, long-term neurological manifestations of COVID-19 are increasingly being reported. The long-term sequelae of COVID-19-related leukoencephalopathy, however, remain unclear. Here, we present long-term neuroimaging follow-up in two cases of COVID-19-related leukoencephalopathy. The two cases demonstrate the utility of brain MRI for evaluating neurologic symptoms in critically ill patients with COVID-19, for diagnosis of underlying neural injury and prognostication of future recovery. The presence of leukoencephalopathy may result in chronic neurologic manifestations and may represent a poor prognosticator of neurologic recovery. The presence of leukoencephalomalacia on follow-up neuroimaging is potentially an indicator of irreversible white matter damage, which may be associated with more severe chronic deficits.
Keywords : COVID-19, Leukoencephalopathy, Chronic, Neuroimaging, MRI
Introduction
Neurological injury related to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been widely reported, including ischemic injury, intracranial hemorrhage, encephalopathy, leukoencephalopathy, and central/peripheral neuropathies [1–3]. Possible mechanisms of neurologic involvement include direct neurotropism, immune-mediated cytokine storm, coagulopathy or vasculopathy, hypoxia, and prolonged illness and hospitalization [4–6]. Some of these mechanisms are also hypothesized to contribute to white matter injury [2, 7]. The long-term sequelae of COVID-19-related leukoencephalopathy, however, remain unclear. As long-term neurological manifestations of COVID-19 are increasingly being reported, there is increasing to better understand the evolution of COVID-19-related neurological changes. Here, we present long-term neuroimaging follow-up in two cases of COVID-19-related leukoencephalopathy.
Case 1
A 63-year-old male with a history of obesity, obstructive sleep apnea, type II diabetes, hypertension, and chronic kidney disease presented to our hospital in early April 2020 for respiratory failure from COVID-19 infection. The patient was intubated in the emergency room and underwent continuous venovenous hemofiltration for acute renal failure. The patient was extubated 18 days after presentation and underwent percutaneous endoscopic gastrostomy and tracheostomy. The patient continued to be encephalopathic during his hospital course with limited motor function and cognitive response, exhibiting only facial grimace to pain, upper extremity flexion to noxious stimuli, and spontaneous blinking. Brain MRI was obtained on hospital day 30 and showed bilateral symmetric multifocal T2/FLAIR hyperintensity and restricted diffusion within the centrum semiovale, corona radiata, periventricular white matter, and corpus callosum (Fig. 1A–C). There was relative sparing of the subcortical U-fibers, cerebral cortex, and deep brain structures. There was no evidence of acute infarction or hemorrhage on susceptibility-weighted image (SWI). The patient was subsequently discharged to a rehab facility without significant change in neurologic status. The patient’s renal function returned to normal.
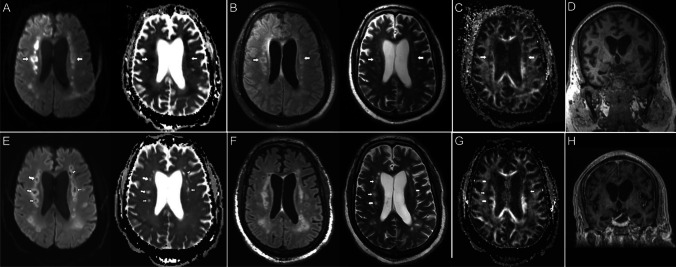
Fig. 1.
Brain MR images of a critically ill patient (case 1) with acute COVID-19 infection (A–D) and follow-up brain MRI 7-month post-discharge (E–H). A Paired axial diffusion-weighted image (DWI) and apparent diffusion coefficient (ADC) map show symmetric foci of restricted diffusion involving the deep white matter of both cerebral hemispheres (arrows). B Paired axial FLAIR and T2 images through the same level show increased T2/FLAIR hyperintensity corresponding to the regions of restricted diffusion (arrows). C Axial fractional anisotropy map shows focal disruption of white matter tracts in the regions of diffusion restriction (arrows). D Coronal T1 image shows mild ventricular prominence suggestive of mild diffuse brain parenchymal volume loss. E Paired axial diffusion-weighted image (DWI) and apparent diffusion coefficient (ADC) map on follow-up MRI both show high signal in the deep white matter of both cerebral hemispheres, consistent with T2 shine-through (arrows). F Paired axial FLAIR and T2 images through the same level show evolution of T2/FLAIR hyperintensity in bilateral subcortical and periventricular white matter with new multifocal cystic changes, consistent with cystic leukoencephalomalacia (arrows). G Axial fractional anisotropy map shows persistent disruption of white matter tracts in the regions of previous diffusion restriction (arrows). H Coronal T1 image shows mild interval increase in size of the ventricular system suggestive of subtle central predominant parenchymal volume loss
At 7-month follow-up, the patient continued to be nonverbal but exhibited increased alertness. He was able to respond to commands with head shakes and nods. His motor function was limited to the right upper extremity and left lower extremity. Follow-up MRI was performed as this time and demonstrated persistent T2/FLAIR hyperintensity involving the supratentorial white matter (Fig. 1E, F). There were also new multifocal cystic changes involving the centrum semiovale and corona radiata with subtle increase in ventricular prominence, suggestive of central predominant parenchymal volume loss (Fig. 1E, G, H). These findings are compatible with cystic leukoencephalomalacia. The patient currently resides at a skilled nursing facility without significant improvement in his neurological condition.
Case 2
A 58-year-old male with a history of obesity, obstructive sleep apnea, type II diabetes, and hypertension presented to our hospital in April 2020 for respiratory failure from acute COVID-19 infection. The patient was intubated in the emergency department and required hemodialysis due to acute on chronic renal failure. The patient was weaned to capped tracheostomy tube a month later. The patient continued to exhibit altered mental status and was unable to follow commands, for which a brain MRI was obtained on admission day 35. The MRI demonstrated bilateral confluent and symmetric T2/FLAIR hyperintensity with restricted diffusion in the supratentorial subcortical white matter with sparing of the subcortical U-fibers (Fig. 2A–C). There was no evidence of acute infarction or hemorrhage on SWI. The patient demonstrated minimal improvement in neurologic status at the time of discharge. There was mild improvement in eye tracking and following commands. Motor strength was 1 out of 5 throughout, with inability to perform fine motor tasks.
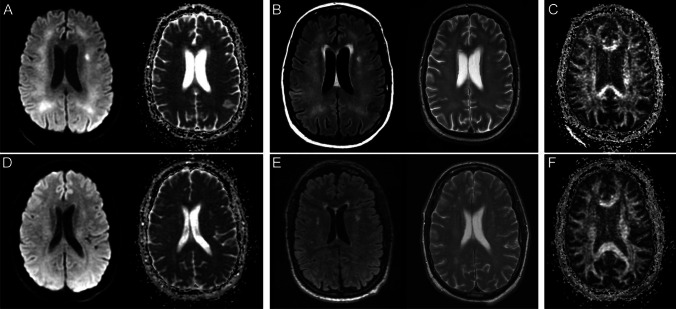
Fig. 2.
Brain MR images of a critically ill patient (case 2) with acute COVID-19 infection (A–C) and follow-up brain MRI 11-month post-discharge (D–F). A Paired axial diffusion-weighted image (DWI) and apparent diffusion coefficient (ADC) map show confluent restricted diffusion in the bilateral supratentorial white matter. B Paired axial FLAIR and T2 images through the same level show confluent symmetric T2/FLAIR hyperintensity in the supratentorial white matter with relative sparing of the subcortical U-fibers, corresponding to the areas of restricted diffusion. C Axial fractional anisotropy map shows no associated disruption of white matter tracts. D Paired axial diffusion-weighted image (DWI) and apparent diffusion coefficient (ADC) map on follow-up brain MRI show resolution of restricted diffusion in the supratentorial white matter. E Paired axial FLAIR and T2 images on follow-up MRI show resolution of confluent T2/FLAIR hyperintensity in the supratentorial white matter with scattered multifocal T2/FLAIR hyperintense foci in the periventricular and subcortical white matter, which may reflect sequelae of leukoencephalopathy. There was no evidence of leukoencephalomalacia. F Axial fractional anisotropy map shows no associated disruption of white matter tracts
This patient presented for follow-up 11 months later and demonstrated substantial improvement in his neurological status with mild residual deficits of executive cognitive function. The patient’s motor strength improved to 3 out of 5 throughout. He continued to have difficulty with fine motor tasks and was wheelchair dependent. Follow up brain MRI obtained at this time demonstrated resolution of confluent T2/FLAIR hyperintensity in the white matter with persistent scattered foci of T2/FLAIR hyperintensity in the periventricular and subcortical white matter, likely reflecting sequelae of prior leukoencephalopathy (Fig. 2D–F). The previously seen restricted diffusion involving the supratentorial white matter had also resolved. No new abnormality was noted on the follow-up examination. There was no evidence of leukoencephalomalacia or appreciable brain parenchymal volume loss. The patient continued to attend physical and occupational therapy sessions.
Discussion
The two cases here highlight that COVID-19-related leukoencephalopathy is not only an acute neurologic condition but can be associated with long-term neurologic impairment. Clinicians should be aware of this chronic neurologic condition, particularly in patients who were critically ill due to COVID-19 and continue to demonstrate chronic neurologic manifestations. Furthermore, brain MRI is an important tool to help work-up the underlying cause of acute and chronic neurologic symptoms in critically ill patients with COVID-19.
The restricted diffusion and T2/FLAIR hyperintensity in the supratentorial white matter during acute COVID-19 infection may reflect hypoxic ischemic damage and potentially cell death [8]. The persistent white matter changes seen on follow-up brain MRI, months after the initial insult, are suggestive of permanent white matter injury and glial/axonal loss, which likely underlie the chronic neurological deficits in both patients. One important difference was that case 1 had focal disruption of white matter tracts in the regions of diffusion restriction on fractional anisotropy and developed multifocal cystic changes, which could account for the more pronounced clinical neurologic deficits.
Although acute COVID-19 leukoencephalopathy with nodular and ring-shaped restricted diffusion have been reported, this is the first report of multifocal cystic leukoencephalomalacia as a chronic sequela of COVID-19-related leukoencephalopathy [2, 7, 9]. The presence of this finding, which is indicative of white matter necrosis with resulting gliosis and irreversible axonal damage, may be a poor prognosticator for neurologic recovery.
Hypoxic-ischemic and toxic/metabolic leukoencephalopathy are hypothesized as the most likely etiologies of white matter injury in these two patients, given the history of respiratory failure requiring intubation and metabolic derangements from renal failure. Relative sparing of the subcortical U-fibers is classically seen in hypoxic and metabolic-related leukoencephalopathies, but other causes including direct neurotropism, inflammatory/autoimmune-related causes, endotheliitis, and vasculitis have been hypothesized as the underlying etiology of acute COVID-19 leukoencephalopathy [7, 9, 10]. The true underlying mechanisms of COVID-19-related demyelinating injury is likely different for various clinical presentations, which should be considered when interpreting neuroimaging findings. Larger prospective study is needed to better characterize long-term neuroimaging changes related to COVID-19 infection.
Acknowledgements
None
Authors’ contributions
Please refer to the author contribution list included in this submission for details on each authors contribution.
Funding
There was no funding for this research project.
Data availability
Deidentified information of the two patients can be made available if required.
Code availability
No custom-made code or commercially available software code was used.
Declarations
Conflict of interest
None of the authors have any conflict of interest. The authors have no disclosures to declare.
Ethics approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individuals included in the study.
Consent for publication
All authors provide consent for publication.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kremer S, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297(2):E242–E251. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang M, Buch K, Li MD, Mehan WA, Jr, Lang AL, Leslie-Mazwi TM, Rincon SP. Leukoencephalopathy associated with severe COVID-19 infection: sequela of hypoxemia? AJNR Am J Neuroradiol. 2020;41(9):1641–1645. doi: 10.3174/ajnr.A6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson P, Alshafai L, Krings T. Neuroimaging findings in patients with COVID-19. AJNR Am J Neuroradiol. 2020;41(8):1380–1383. doi: 10.3174/ajnr.A6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moonis G, Filippi CG, Kirsch CFE, Mohan S, Stein EG, Hirsch JA, Mahajan A (2020) The spectrum of neuroimaging findings on CT and MRI in adults with coronavirus disease (COVID-19). AJR Am J Roentgenol. 2021 Oct;217(4):959–974. [DOI] [PubMed]
- 5.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183(1):16–27 e1. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radmanesh A, Derman A, Lui YW, Raz E, Loh JP, Hagiwara M, Borja MJ, Zan E, Fatterpekar GM. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297(1):E223–E227. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF, Jr, Sabeti P. Neuropathological features of Covid-19. N Engl J Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toledano-Massiah S, Badat N, Leberre A, Bruel C, Ray A, Gerber S, Zins M, Hodel J. Unusual Brain MRI Pattern in 2 Patients with COVID-19 acute respiratory distress syndrome. AJNR Am J Neuroradiol. 2020;41(12):2204–2205. doi: 10.3174/ajnr.A6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarbu N, Shih RY, Jones RV, Horkayne-Szakaly I, Oleaga L, Smirniotopoulos JG. White matter diseases with radiologic-pathologic correlation. Radiographics. 2016;36(5):1426–1447. doi: 10.1148/rg.2016160031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified information of the two patients can be made available if required.
No custom-made code or commercially available software code was used.