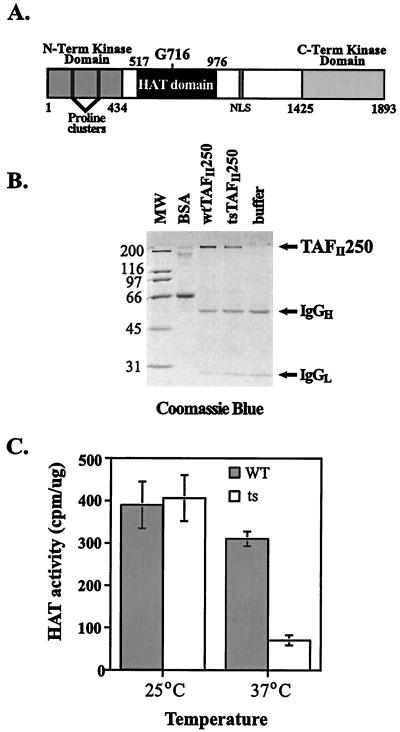
FIG. 1.
ts13 TAFII250 mutant protein displays ts HAT activity in vitro. (A) Schematic diagram of human TAFII250 protein. The positions of known functional domains are indicated. The homologous human amino acid residue mutated in ts13 cells (G716) maps to the putative HAT domain of TAFII250, suggesting that this enzymatic activity is compromised in ts13 cells. Term, terminal; NLS, nuclear localization signal. WT and mutant TAFII250 were purified from baculovirus-infected Sf9 extracts using immunoprecipitation techniques, run on SDS-polyacrylamide gels, and visualized by Coomassie blue staining to show purity. As a control, Sfa lysis buffer was subjected to immunoprecipitation. Positions of the immunoglobulin heavy (IgGH) and light (IgGL) chains are indicated. Positions of molecular weight standards (MW) are indicated in kilodaltons at the left. BSA, bovine serum albumin. (C) Purified TAFII250 proteins described for panel B were added to liquid HAT assays containing purified calf thymus histones and [3H]acetyl coenzyme A and incubated at 25°C (six experiments with 5 ≤ n ≤ 10) or 37°C (eight experiments with 5 ≤ n ≤ 10). The amount of acetylated histones was measured by liquid scintillation.