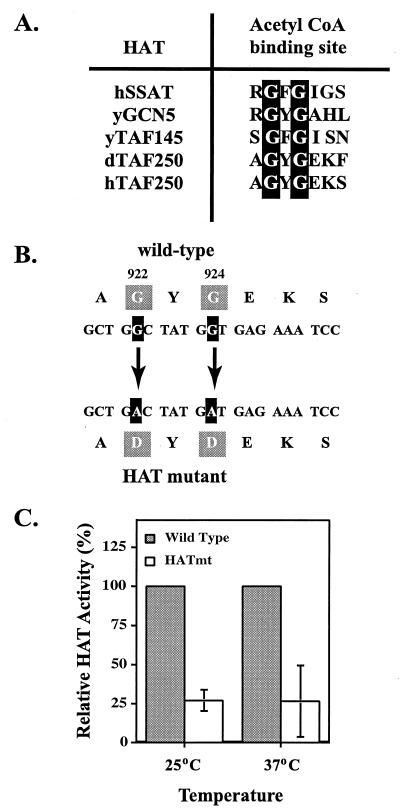
FIG. 2.
Mutation of a putative acetyl coenzyme A (CoA) binding site compromises TAFII250 HAT activity. (A) A consensus sequence for an acetyl coenzyme A binding site has been identified in human (h), yeast (y), and Drosophila (d) HAT proteins including TAFII250 as shown. (B) Two conserved glycine residues present in human TAFII250 were mutated, as indicated, to aspartic acid (G922A and G924A) in order to ablate acetyl coenzyme A binding. (C) The TAFII250 HAT mutant described above and WT TAFII250 were expressed and immunoaffinity purified from recombinant baculovirus-infected Sf9 cells. The HAT activity of the purified WT and HAT mutant proteins was determined at 25°C (six experiments with 5 ≤ n ≤ 10) and 37°C (five experiments with 5 ≤ n ≤ 10) by liquid HAT assays. The relative HAT activity for the HAT mutant TAFII250 is expressed as a percentage of the activity detected with the WT protein (given a value of 100%) at each temperature.