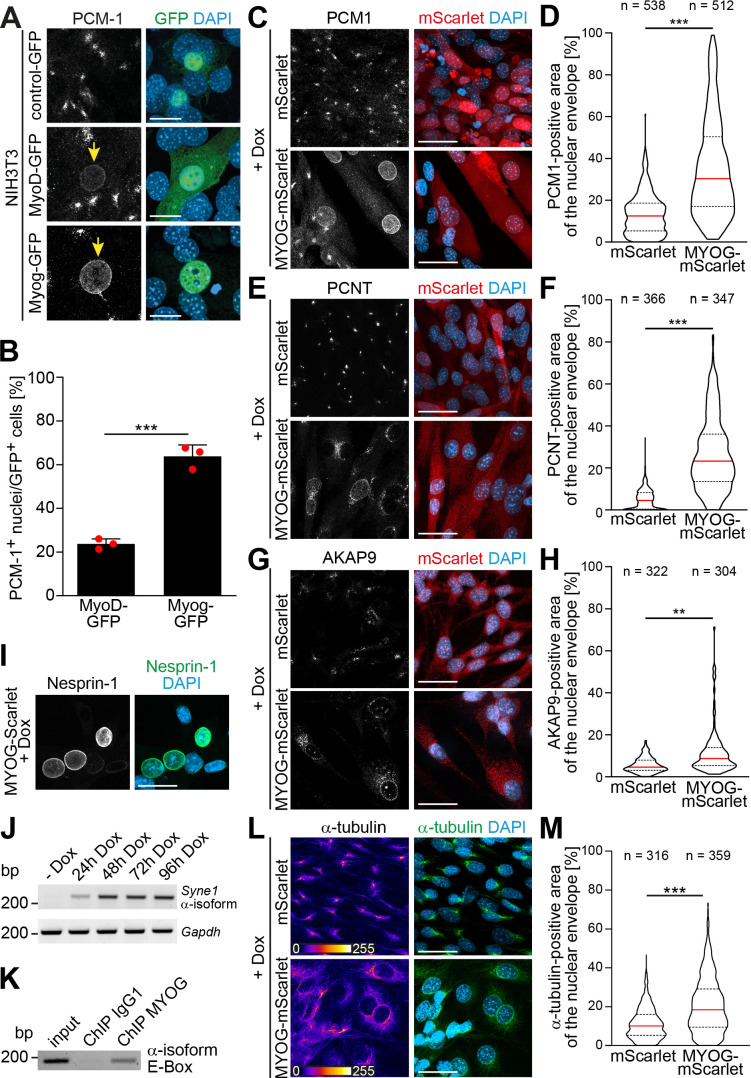
Figure 2. Myogenin expression is sufficient to induce nuclear envelope microtubule-organizing center (NE-MTOC) formation in non-muscle cells.
(A) NIH3T3 fibroblasts were transfected with constructs encoding GFP, MyoD-GFP or myogenin-GFP (Myog-GFP). After three days, PCM-1 localization was assessed by immunostaining. Arrows indicate nuclei of transfected cells which have recruited PCM‑1. Scale bars: 10 µm. (B) Quantification of (A) demonstrating that myogenin induces nuclear envelope localization of PCM‑1 more efficiently than MyoD. Data are represented as individual biological replicates (n = 3), together with mean ± SD. ***: p < 0.001; 95% CI of difference Myog-GFP vs. MyoD-GFP = 30.99% to 49.22%. n = 3. (C-H) NIH3T3 Tet-ON mScarlet or MYOG-2A-mScarlet (MYOG-mScarlet) cells were treated with doxycycline (Dox) for three days. After immunostaining, nuclear envelope localization of PCM-1 (C-D), PCNT (E-F), and AKAP9 (G-H) was analyzed and quantified. Data are depicted as violin plots. Red line indicates the median, dotted lines indicate the 25% and 75% percentile. ***: p < 0.001. Scale bars: 20 µm (I) Immunostaining of MYOG-mScarlet cells treated with Dox for three days showing the presence of nesprin‑1α+ nuclei. Scale bars: 20 µm (J) RT-PCR analysis of MYOG-mScarlet cells in the absence of Dox (-Dox) or treated with Dox for the indicated time points demonstrating that nesprin‑1α is upregulated upon myogenin expression. Gapdh was used as equal input control. (K) ChIP-PCR analysis of Dox-treated MYOG-mScarlet cells using an anti-myogenin antibody or an IgG1 control showing that myogenin binds an E-box in the nesprin-1α promoter region. (L-M) Immunostaining of α-tubulin and subsequent quantification of nuclear envelope coverage after 30s of microtubule regrowth following cold-induced microtubule depolymerization in mScarlet or MYOG-mScarlet cells treated with Dox for three days. Data are depicted as violin plots. Red line indicates the median, dotted lines indicate the 25% and 75% percentile. ***: p < 0.001. Scale bars: 20 µm. N numbers indicate total number of analyzed nuclei pooled from three biological replicates.
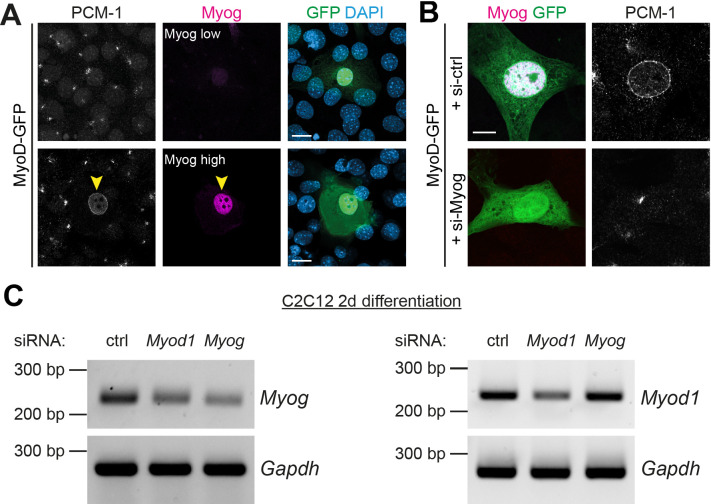
Figure 2—figure supplement 1. Myogenin is required for MyoD-induced nuclear envelope recruitment of PCM-1.
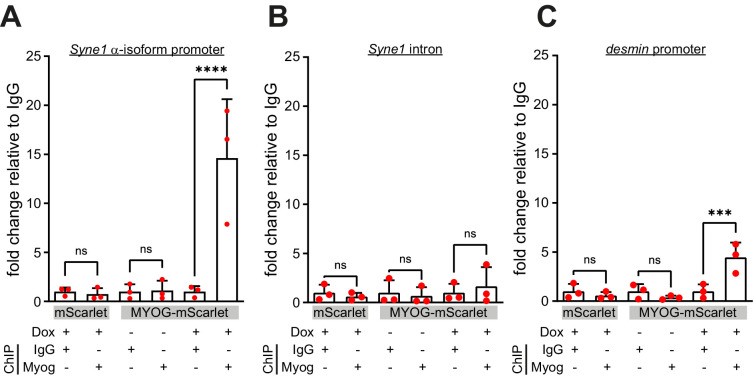
Figure 2—figure supplement 2. Myogenin targets are specifically precipitated in induced MYOG-mScarlet cells.
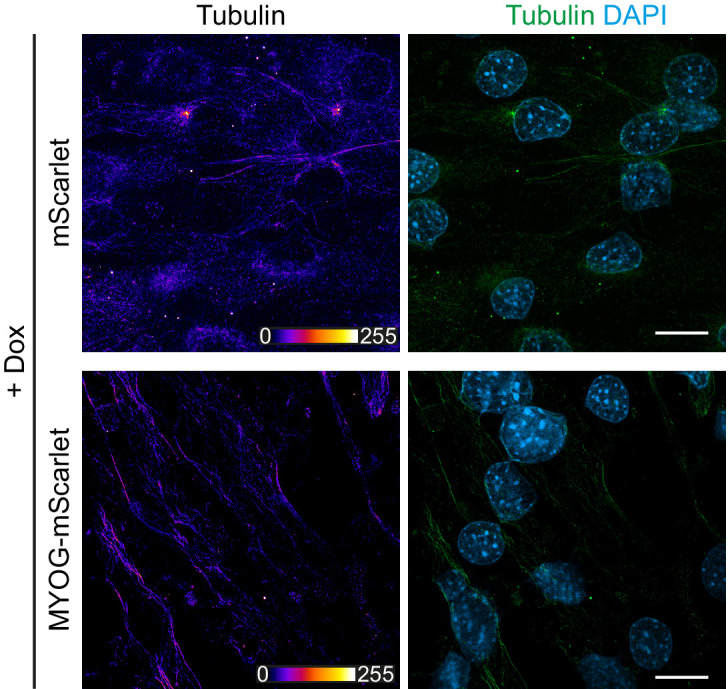
Figure 2—figure supplement 3. Microtubule depolymerization in mScarlet and MYOG-mScarlet cells.