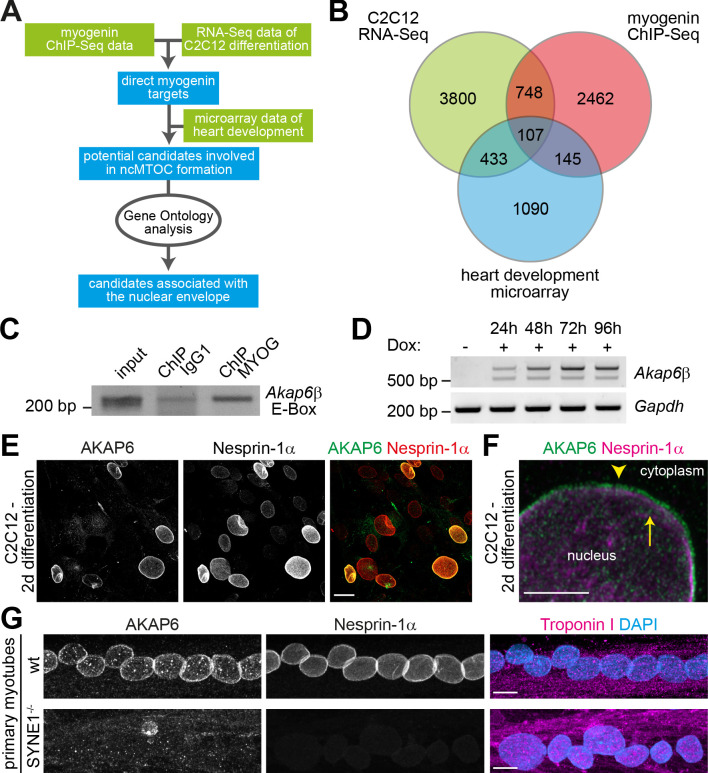
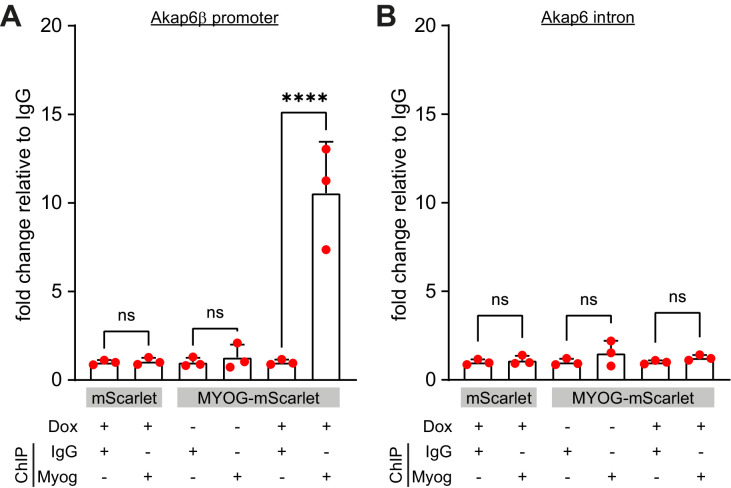
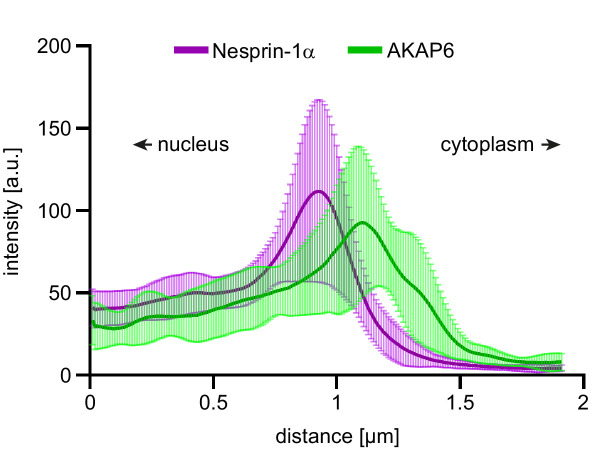
Figure 4. The nesprin-1α interaction partner AKAP6 is a potential mediator of myogenin-induced nuclear envelope microtubule-organizing center (NE-MTOC) formation.
(A) Scheme illustrating the bioinformatics workflow used to identify potential myogenin downstream candidates. (B) Venn diagram depicting the numbers of genes matching criteria for the individual data sets and for intersection of data sets. Criteria for myogenin ChIP-seq data (red): Genes where myogenin binding was detected at the promoter region; criteria for C2C12 RNA-seq data (green) and for microarray data of rat heart development (blue): upregulated genes. (C) ChIP-PCR analysis of doxycycline (Dox)-treated MYOG-mScarlet cells using an anti-myogenin antibody or an IgG1 control showing that myogenin binds an E-box in the Akap6β promoter region. (D) RT-PCR analysis of MYOG-mScarlet cells in the absence of Dox (-Dox) or treated with Dox for the indicated time points demonstrating that Akap6β is upregulated upon myogenin expression. The two bands for Akap6β derive from alternative splicing of the first exon of Akap6β, which results in an ~200 bp insertion in the 5’ untranslated region. Gapdh was used as equal input control. Please note that the same samples and Gapdh control were used as in Figure 2J. (E) C2C12 cells were differentiated for 2 days, and immunostaining shows that all AKAP6+ nuclei are also nesprin-1α+. Scale bar: 20 µm. (F) High-resolution Airyscan image of (E). Arrowhead indicates AKAP6 localized at the cytoplasmic side of nesprin-1α signal. Arrow marks nesprin-1α that is localized at the nuclear side of AKAP6 signal. Scale bar: 0.5 µm. (G) Myoblasts from healthy donors (wt) and from patients carrying a mutation in the SYNE1 gene (SYNE1-/-) were differentiated for 4 days. Immunostaining analysis showed that loss of nesprin-1α is associated with loss of AKAP6 from the nuclear envelope in differentiated myotubes (troponin I). Scale bars: 10 µm.