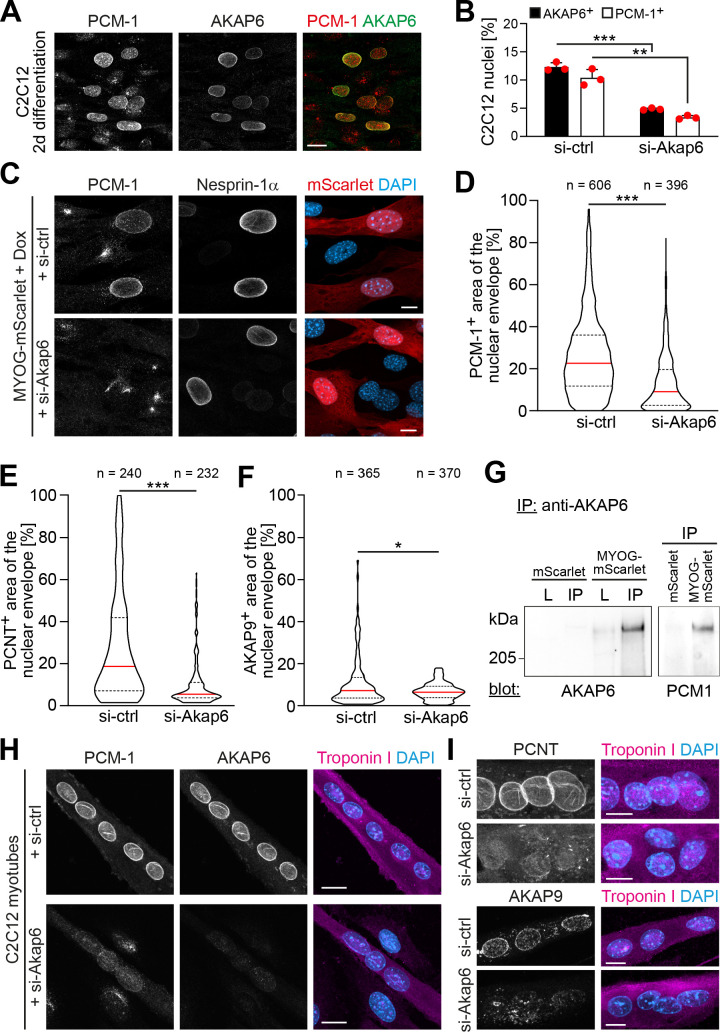
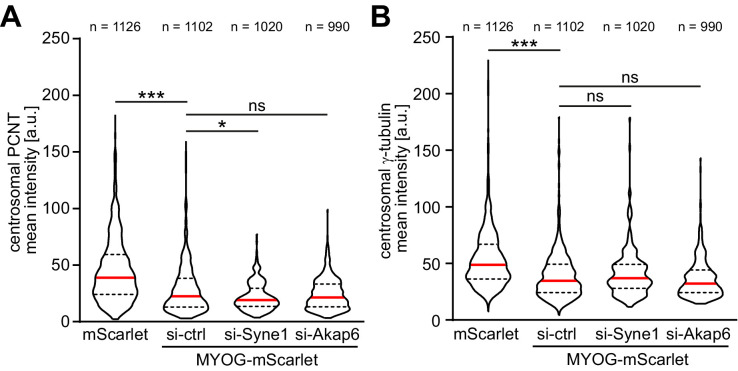
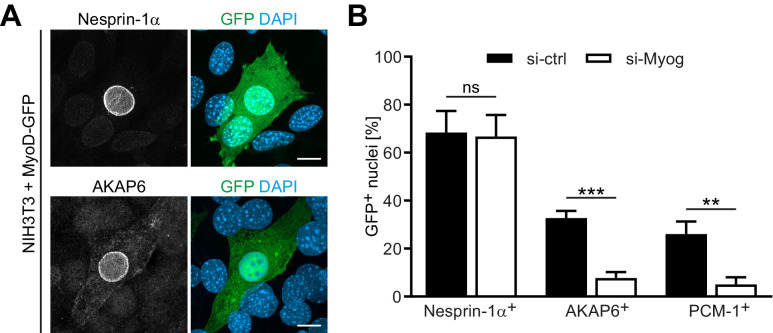
Figure 5. AKAP6 is required for the nuclear envelope localization of microtubule-organizing center (MTOC) proteins.
(A) C2C12 cells were differentiated for 2 days. Immunostaining shows that all PCM-1+ nuclei are also AKAP6+. (B) Quantification of AKAP6+ and PCM-1 nuclei in C2C12 cells treated with negative control (si-ctrl) or Akap6 (si-Akap6) siRNA after 2 days of differentiation indicates that AKAP6 is required for nuclear envelope localization of PCM-1. Data are represented as individual biological replicates (n = 3), together with mean ± SD. 95% CI = 6.21% to 8.74%; 95% CI = 4.63% to 9.43%. (C) MYOG-mScarlet cells were treated with si-ctrl or si-Akap6 and subsequently treated with doxycycline (Dox) for 3 days. Image analysis revealed that myogenin-induced localization of PCM-1 to the nuclear envelope is AKAP6-dependent. (D) Quantification of (C). (E, F) Quantification of PCNT (E) and AKAP9 (F) nuclear coverage in Dox-stimulated MYOG-mScarlet cells treated with si-ctrl or si-Akap6. (G) Co-immunoprecipitation (IP) of PCM-1 from MYOG-mScarlet but not from mScarlet lysate (L) using an anti-AKAP6 antibody. (H, I) Enriched C2C12 myotubes (troponin I) were transfected with si-ctrl or si-Akap6 and immunostaining demonstrates that AKAP6 is required for maintaining nuclear envelope localization of PCM-1 (H) as well as PCNT and AKAP9 (I). Scale bars (A, H) 20 µm, (C, I) 10 µm. Data (D–F) are shown as violin plots. The red line indicates the median, and dotted lines indicate the 25% and 75% percentile. N numbers indicate the total number of analyzed nuclei pooled from three biological replicates. *p<0.05.; **p<0.01; ***p<0.001.
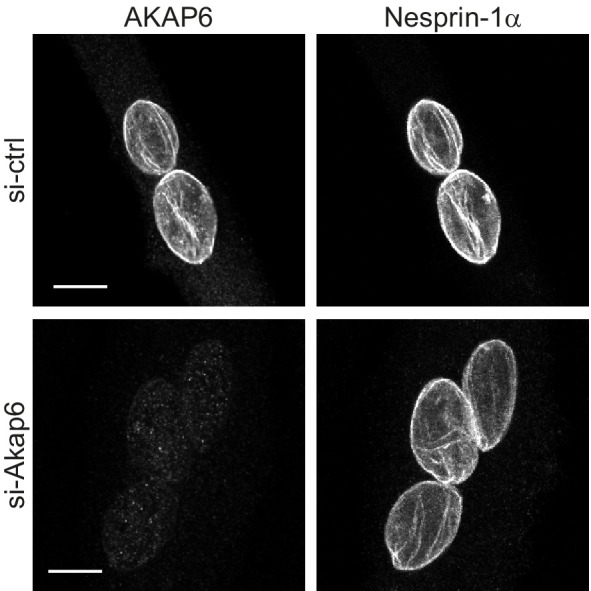
Figure 5—figure supplement 1. AKAP6 depletion does not affect nesprin-1α.