Fig. 2.
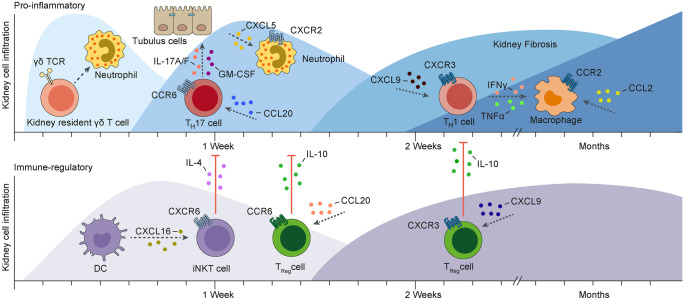
The time-dependent changes of pro-inflammatory and immune-regulatory functions of leukocyte subsets during the course of experimental crescentic glomerulonephritis (NTN) are shown. NTN is induced by an injection of a heterologous sheep-anti-mouse serum and, within a few hours, resident γδ T cells activate neutrophils. Shortly after, the autologous pro-inflammatory immune response is firstly mediated by the recruitment of CCR6 expressing T helper 17 (TH17) cells in response to local CC-chemokine ligand (CCL)20 production. These infiltrating TH17 cells produce pro-inflammatory cytokines, i.e., interleukin (IL)-17A, IL-17F and GM-CSF, leading to the recruitment and activation of tissue disruptive neutrophils. Later, the recruitment of CXCR3 expressing T helper 1 (TH1) cells in response to local CXCL9 production prevails. These infiltrating TH1 cells produce cytokines such as interferon-γ (IFNγ) and tumor necrosis factor α (TNFα), which are potent activators of macrophages, leading to their releasing injurious mediators such as nitric oxide. Simultaneously, during the first days of NTN, dendritic cells (DCs) attenuate crescentic glomerulonephritis by attracting regulatory invariant natural killer T (iNKT) cells via the CXC-chemokine ligand (CXCL) 16–CXCR6 axis and these cells produce IL-4 thereby reducing destructive TH17 cell responses. At a later stage, CCR6+ and CXCR3+ regulatory T (Treg) cells are recruited into the inflamed kidney, respectively and protect against an overwhelming TH17 cell- and TH1 cell-mediated immune response, at least partly through the local production of IL-10. CCR, CC-chemokine receptor; CXCR, CXC-chemokine receptor; TCR, T cell receptor; GM-CSF, granulocyte–macrophage colony-stimulating factor