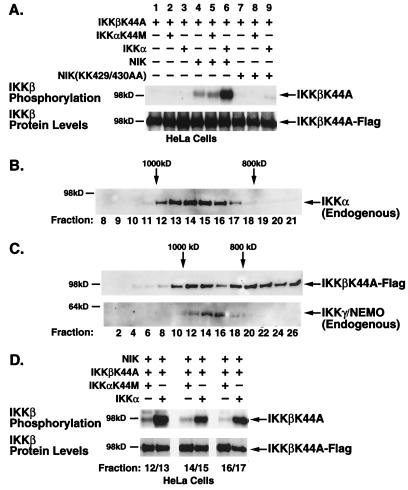
FIG. 4.
NIK-induced phosphorylation of IKKβ requires catalytically active IKKα. (A) HeLa cells were transfected with IKKβK44A alone or with either wild-type or kinase-inactive NIK in combination with wild-type or kinase-inactive IKKα. Cells were lysed 48 h posttransfection. Signalsomes were immunoprecipitated with anti-IKKγ/NEMO antibodies and subjected to an in vitro kinase assay followed by heat dissociation in 10% SDS. IKKβK44A substrates were selectively immunoprecipitated from the disrupted complexes by a second immunoprecipitation with anti-Flag M2-agarose. (B) Unstimulated and TNF-α-stimulated (5 min) HeLa cell lysates were subjected to FPLC size fractionation on a Superose 6 column. Fractions were collected, separated by SDS-PAGE, and immunoblotted with anti-IKKα antibodies to identify fractions containing the endogenous signalsome (fractions 12 to 17, ∼900 kDa). (C) HeLa cells, transfected with Flag-tagged, kinase-inactive IKKβ, NIK, and either kinase-proficient or kinase-defective IKKα, were lysed and size fractionated by FPLC. Fractions were separated by SDS-PAGE followed by immunoblotting with anti-Flag or anti-IKKγ antibodies. (D) Fractions corresponding to those containing the endogenous IKK signalsome, as identified by anti-IKKα and anti-IKKγ antibodies, were collected, pooled, immunoprecipitated, and subjected to an in vitro kinase assay as described for Fig. 2. The level of phosphorylated IKKβ-K44A is shown in the upper panel; the levels of protein as determined by anti-Flag immunoblotting are shown in the lower panel.