Figure 5.
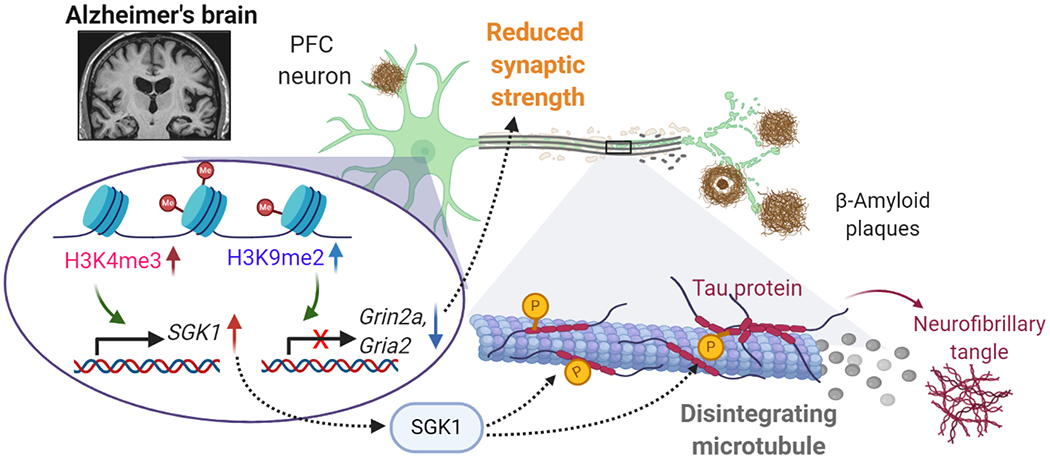
Schematic diagram illustrating the potential epigenetic mechanisms underlying gene dysregulation and synaptic deficits in PFC of AD. The elevation of repressive histone mark H3K9me2 in AD leads to the downregulation of genes involved in synaptic transmission, such as AMPAR subunit Gria2 and NMDAR subunit Grin2a, resulting in the reduction of synaptic strength256. On the other hand, the elevation of permissive histone mark H3K4me3 in AD leads to the upregulation of genes involved in cell stress, such as serum- and glucocorticoid-inducible kinase 1 (SGK1), resulting in hyperphosphorylation of tau, disintegration of microtubules and disruption of vital protein transport260. Targeting histone methytransferases to normalize histone modification or key target genes is the potential new therapeutic strategy for treating synaptic and cognitive deficits in AD.
