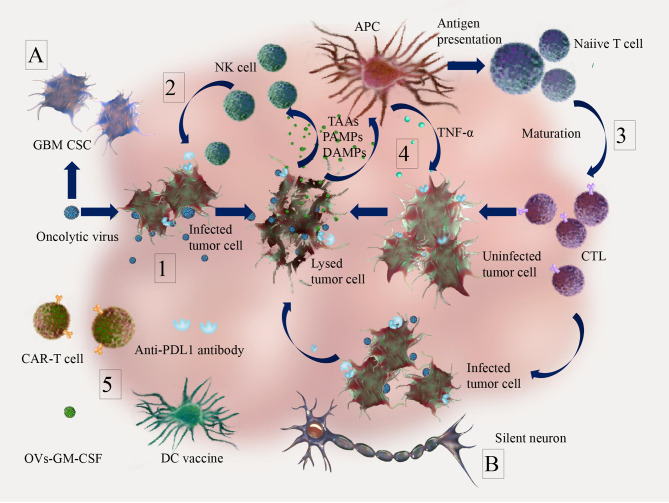
Figure 2.
Anti-tumor effects of oncolytic virus (OV) and combination therapy in brain tumor. 1. OVs can selectively or preferentially infect tumor cells and induce tumor lysis. 2. Innate immune response. Tumor cell lysis due to OVs infection can cause the release of tumor associated antigens (TAAs), cell-derived damage-associated molecular patterns (DAMPs) and viral pathogen-associated molecular patterns (PAMPs), which can recruit dendritic cells (DCs) and innate lymphoid cells (e.g. NK cells) for early clearance of virus-infected cells; 3. Adaptive immune response. The release of TAAs, DAMPs, PAMPs, pro-inflammatory cytokines and chemokines by lysed tumor cells can trigger activation of antigen presenting cells (APCs) and promote the priming of cellular mediated immune responses (CTL infiltration); 4. OVs infection leads to the release of TAAs, PAMPs and DAMPs, which can induce innate immune responses (e.g. secretion of TNF-α) against not only infected tumor cells, but also uninfected tumor cells through bystander effects; 5. Infection and replication of oncolytic viruses in tumors can activate anti-tumor immunity and turn “cold” into “hot” tumors, which make combination therapies such as immune checkpoint inhibitors (e.g. PD-1/PD-L1 inhibitor), adoptive cell therapy (e.g. CAR-T), tumor vaccines (e.g. DC vaccine) and immunotherapeutic modulators (e.g. GM-CSF, which can enhance the activation of NK cells and CD8-mediated T cell response) more effective. For glioma specifics, (A) antiviral innate immunity pathways (IFN pathway, TLR pathway) are reduced in glioblastoma cancer stem cells (GBM CSC), which contributes to the tumor cell specificity of OVs. (B) Due to the isolated location surrounded by mitotically silent normal neurons, malignant gliomas may be particularly suitable for treatment with OVs, which require active cell cycles for their replication.