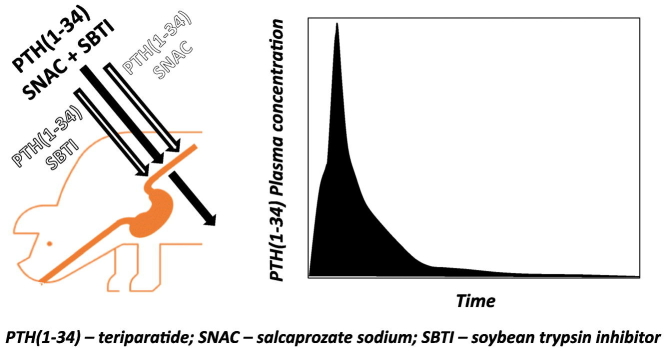
Abstract
Oral delivery of peptides and proteins is hindered by their rapid proteolysis in the gastrointestinal tract and their inability to permeate biological membranes. Various drug delivery approaches are being investigated and implemented to overcome these obstacles. In the discussed study conducted in pigs, an investigation was undertaken to assess the effect of combination of a permeation enhancer – salcaprozate sodium, and a proteolysis inhibitor – soybean trypsin inhibitor, on the systemic exposure of the peptide teriparatide, following intraduodenal administration. Results demonstrate that this combination achieves significantly higher Cmax and AUC (~10- and ~20-fold respectively) compared to each of these methodologies on their own. It was thus concluded that an appropriate combination of different technological approaches may considerably contribute to an efficient oral delivery of biological macromolecules.
Keywords: Oral delivery, Permeation enhancer, Pharmacokinetics, Salcaprozate sodium, SNAC, Soybean trypsin inhibitor, Teriparatide
Abbreviations: SBTI, soybean trypsin inhibitor; SNAC, salcaprozate sodium; GIT, gastrointestinal tract; hPTH(1–34), teriparatide; PK, pharmacokinetics; PTH, parathyroid hormone
Graphical abstract
Highlights
-
•
Soybean trypsin inhibitor (SBTI) protects hPTH(1–34) from proteolysis in the intestine.
-
•
SNAC/SBTI combination significantly raises plasma exposure of oral hPTH(1–34).
-
•
Oral formulation hPTH(1–34)/SNAC/SBTI befits the PK profile for osteoporosis treatment.
-
•
Endoscopic intraduodenal delivery in pigs enables investigation of absorption mechanisms.
1. Introduction
The therapeutic reliance on peptide and protein drugs has expanded in the last three decades and continues to grow at an ever-fast pace (Fosgerau and Hoffmann, 2015). Their potency and specific mode of action result in predictable responses, low toxicity, and minimal nonspecific and/or drug-drug interactions. All these characteristics make them particularly attractive as active drug entities.
Oral route of administration is generally considered to be the preferred one for patients due to convenience, safety, and acceptance (Lennernäs et al., 2007). For people suffering from chronic diseases requiring frequent injections, such as diabetes, growth hormone deficiency and osteoporosis, the oral route of administration as a substitute to injection might be particularly attractive and is likely to increase compliance, adherence and improve the quality of life. However, oral delivery of biological drugs is a challenging undertaking given that their absorption is hampered by the inability to permeate the lipophilic membrane of the cells lining the gastrointestinal tract (GIT) due to their hydrophilic nature, large molecular size, and significant degradation by enzymes in the GIT (Smart et al., 2014; Brayden et al., 2020). These factors make the oral bioavailability of biological drugs negligible and clinically irrelevant. Thus, significant efforts are undertaken to enable the transfer of macromolecules from the parenteral to oral administration route.
The research in the field of oral delivery is driven by the utilization of several methods, alas with only limited clinical success. These methods can be categorized into two main approaches (Aguirre et al., 2016): 1) prevention of proteolysis in the GIT, thereby enabling peptide and protein molecules to reach the membrane of epithelial cells intact, and 2) enhancement of the molecule's permeation through the epithelial lining. Prevention of enzymatic degradation can be achieved by chemical modification of the drug molecule, inhibition of gastrointestinal peptidases, or physical protection of the drug. Permeation enhancement can be attained by the utilization of transcellular or paracellular permeation enhancing methods, as well as by chemical modifications of the drug molecule.
A combination of these two main approaches enables further advances towards reaching the goal of oral delivery of biological drugs. This concept has been implemented, with promising results, by several clinical-stage biotech companies. Oramed Pharmaceuticals Inc. successfully enhances oral bioavailability of insulin by co-administration with a protease inhibitor and a Ca+ chelating agent, which increases paracellular permeability (Eldor et al., 2010; Eldor et al., 2013). Enhanced bioavailability of salmon calcitonin is achieved by Enteris BioPharma Inc. following co-administration with a pH modifying agent – citric acid, inhibiting the activity of digestive enzymes, and a paracellular permeation enhancer – acylcarnitine (Welling et al., 2014). Several additional clinical studies utilizing combinations of the two approaches can also be found in the literature (Brayden et al., 2020).
Published data specifically evaluating this combination approach is lacking. Therefore, the current investigation was conducted with the objective to study the combined effect of the two approaches on the extent of systemic absorption following per oral administration of the peptide teriparatide (hPTH(1–34)).
hPTH(1–34) is a bioactive peptide, comprised of the 34 amino acids from the N-terminus of human parathyroid hormone (PTH). PTH is important for calcium homeostasis. Exogenously administered hPTH(1–34) is used in the treatment of different forms of osteoporosis (Hock and Gera, 1992) and is being researched as a treatment for hypoparathyroidism (Burshtein et al., 2017). Forteo® is the first product utilizing hPTH(1–34) by subcutaneous injection, for the treatment of osteoporosis (Neer et al., 2001).
hPTH(1–34) is rapidly degraded by the GIT proteases pepsin, chymotrypsin, and trypsin as shown by Werle et al. (2006). In their work, it was also shown that other major GIT peptidases (namely: aminopeptidase N, elastase, and membrane-bound peptidases) had a very limited effect on the degradation of hPTH(1–34), making these enzymes not relevant for the time frame of hPTH(1–34) absorption. In addition, with a relatively high molecular weight of 4.12 kDa and very high hydrophilicity of LogP ~ -18, hPTH(1–34) permeability through lipophilic membranes is very low. In the present work, hPTH(1–34) was co-formulated with either a permeation enhancer – salcaprozate sodium (SNAC), a protease inhibitor – soybean trypsin inhibitor (SBTI), or both.
SNAC has been extensively investigated as an intestinal permeation enhancer of orally delivered macromolecules for more than 20 years (Twarog et al., 2019). This research culminated with the recent utilization of SNAC in the Novo Nordisk A/S product Rybelsus®, an oral tablet containing SNAC with a peptide drug semaglutide (Bucheit et al., 2020) and approved by FDA in late 2019. This product is considered to be the first oral combination of a peptide drug with a permeation enhancer, to reach the market.
The permeation enhancement mechanism of action of SNAC has not been fully elucidated. SNAC is known to increase the passive transcellular permeation rate of co-administered active molecules through a transient increase in membrane fluidity of the cells lining the GIT (Alani and Robinson, 2008; Brayden et al., 1997; Hess et al., 2005; Malkov et al., 2002; Twarog et al., 2019; Twarog et al., 2020). Additionally, this permeation enhancement effect requires a high local concentration of SNAC in close proximity to the intestinal wall (Hess et al., 2005; Twarog et al., 2019). The delivered drug must be in this zone of high SNAC concentration (Buckley et al., 2018; McIntyre et al., 2011). The increased permeability effect diminishes rapidly as SNAC's local concentration declines, due to its dilution in fluids present in the lumen of the GIT and its own systemic absorption (Karsdal et al., 2015). As this local effect of SNAC is short-lasting, the time frame for the increase in the permeability of the active molecule is limited. It was also suggested (Buckley et al., 2018) that in the semaglutide formulation SNAC has a dual mode of action in the stomach. In addition to the permeability enhancement effect, it was proposed that SNAC also prevents proteolytic action of pepsin on the semaglutide by elevating the gastric pH. Absorption of hPTH(1–34) following oral administration was shown to be significantly augmented by SNAC in a comprehensive in vivo study in rats undertaken by Emisphere Technologies Inc. (Leone-Bay et al., 2001).
SBTI is a protein found in soybeans, and is a potent inhibitor of the digestive proteolytic enzymes trypsin, chymotrypsin, and elastase (Birk, 1976), the same enzymes which extensively degrade hPTH(1–34). SBTI acts as a competitive substrate, forming a complex with the digestive enzyme, which cannot be dissociated by the enzyme, thereby efficiently blocking its active site and protease activity (Laskowski and Kato, 1980). When released concomitantly with the drug from the formulation, the action of local enzymatic inhibition will prevent the drug from fast degradation. SBTI is not a part of a commercial product. Nevertheless, numerous human trials were completed with SBTI (Hernández-Ledesma and Hsieh, 2017; Burshtein et al. 2018b). Results from these studies indicate that SBTI, at the levels found in the tested formulations, was well tolerated.
The current work was aimed to study the combined effect of per oral co-administration of SNAC and SBTI on the systemic exposure of hPTH(1–34). This approach was tested in vivo in pigs, following direct intraduodenal administration.
2. Materials and methods
2.1. Chemicals
hPTH(1–34) acetate was purchased from Bachem (Bubendorf, Switzerland), L-(tosylamido-2-phenyl) ethyl chloromethyl ketone (TPCK) treated bovine pancreas trypsin (I.U.B. 3.4.21.4; 225 p-toluene-sulfonyl-L-arginine methyl ester (TAME) units/mg solid) and tosyl-L-lysyl-chloromethane hydrochloride (TLCK) treated bovine pancreas chymotrypsin (I.U.B. 3.4.21.1; 61.8 N-Benzoyl-L-tyrosine ethyl ester (BTEE) units/mg solid) were purchased from Worthington Industries (Columbus, OH, US), SBTI (Bowman-Birk type, Mw = 8000 g/mol, 349.6 TAME units/mg solid) was purchased from BBI solutions (Blaenavon, UK), SNAC (assay = 97.0%) was produced by Entera Bio Ltd. (Jerusalem, Israel), lactose was purchased from Sigma (Jerusalem, Israel), magnesium stearate was kindly provided by Merck & Co (Darmstadt, Germany), HPLC grade acetonitrile was purchased from J.T Baker (Avantor Performance Materials, LLC, Radnor, PA, US), water was purified with a tandem RiOs (reverse osmosis)/Milli-Q Gradient A-10 system (Millipore, Molsheim, France), and all other chemicals were of analytical grade.
2.2. Tested formulations
Three different formulations were prepared according to Table 1 (based on Ish-Shalom et al., 2021 and Burshtein et al. 2018a). Formulations were prepared by geometrical mixing of constituents using a mortar and pestle, followed by dry granulation. The granulate was sifted and the fraction between sieves of 14 to 20 Mesh was collected and used in the study. Lactose was added instead of SNAC to the SBTI/hPTH(1–34) formulation as a filler to ensure similar final weight of the tested formulations.
Table 1.
Content of each formulation tested in the study.
| Formulation ID | Quantity in the formulation (%) |
||||
|---|---|---|---|---|---|
| hPTH(1–34) | SNAC | SBTI | Lactose | Mg stearate | |
| SNAC/SBTI/hPTH(1–34) | 0.6 | 84.4 | 14.0 | – | 1.0 |
| SNAC/hPTH(1–34) | 0.6 | 98.4 | – | – | 1.0 |
| SBTI/hPTH(1–34) | 0.6 | – | 14.0 | 84.4 | 1.0 |
hPTH(1–34) – teriparatide (as acetate), SNAC – salcaprozate sodium, SBTI – soybean trypsin inhibitor.
2.3. Dissolution test
A dissolution test of 133 mg of each formulation was performed according to the USP compendial test <711> using apparatus II (Paddle) in 500 mL phosphate buffer pH 6.4 heated to 37 °C, with a stirring rate of 50 RPM. 20 mL samples were withdrawn at time points: 0, 5, 10, 15, and 20 min. After each sampling, the dissolution media was replenished. Samples were filtered through a 13 mm Acrodisc Syringe Filter with 0.45 μm Supor membrane filter (PALL-Life Sciences, Hoegaarden, Belgium) directly into 20 mL volumetric flasks containing 4 mL acetonitrile and filled up to the mark. 100 μL of the obtained solutions were injected into a HPLC-UV.
2.4. In vitro evaluation of hPTH(1–34) protection by SBTI against enzymatic degradation
This experimental design was based on work by Werle et al. (2006). Briefly, 1.0 mL of 50 mM Tris buffer pH 6.5 containing 1.0 mg hPTH(1–34) acetate either with or without 25.1 mg SBTI (resembling the ratio in the formulation) were mixed with 1.0 mL of the Tris buffer containing either 0.5 mg trypsin, 0.9 mg chymotrypsin, or neither. Solutions were incubated at 37 °C with constant shaking. 250 μL samples were withdrawn at: 2, 5, 10, 15, 30, and 60 min, and immediately mixed with 250 μL of 0.5% TFA to stop by the acidic pH the degradative action of the enzymes. Prior to each set of experiments, 125 μL of a hPTH(1–34) containing solution (as described above) was mixed with 125 μL of Tris buffer and 250 μL of TFA 0.5% to imitate the ‘0’ time point. This served as a 100% from which the degradation was calculated. 40 μL were injected into a HPLC-UV. The work was done in duplicate.
2.5. Chromatographic analysis
The separation was performed on a ZORBAX 300SB C-18, 4.6*150 mm column with particle size of 3.5 μm (Agilent Technologies Inc., Santa Clara, CA, US; further referred to as Agilent), protected by a guard column. The column temperature was maintained at 40 °C and the autosampler at 5 °C. Elution of hPTH(1–34) at ~ 8 min (Supplementary Fig. S1) was obtained with flow of 1.0 mL/min of mobile phase comprised of a 1:3 mixture of acetonitrile: 0.2 M sulfate buffer pH 2.3; run time of 15 min. Detection was performed at 214 nm. The work was performed on the HPLC system Agilent 1100 series coupled with DAD detector Agilent 1100 series (Agilent). Results were obtained on the ChemStation for LC 3D systems, Rev.B.04.03-SP1 [87] (Agilent). The working concentration range of 0.5–0.001 mg/mL was shown to be linear with R2 > 0.997.
2.6. Ethics
All surgical and experimental procedures were reviewed and approved by the Israeli National Council for Animal Experimentation (study license number: IL-14-12–362) and comply with the 8th edition (Animals 2011) of the guidelines of the National Institutes of Health for the care and use of laboratory animals.
2.7. Animal model
The study was performed on five female domestic pigs (sus scrofa domesticus) with a mean weight of 46 kg, obtained from ‘Yaar Shivuk’, I'billin, Israel. All pigs received each of the three formulations, with a washout period of at least a week between the experiments. Formulations were administered in the following order: SNAC/SBTI/hPTH(1–34) formulation, SNAC/hPTH(1–34) formulation and SBTI/hPTH(1–34) formulation. 48 h before each experiment, animals were fed a liquid diet and fasted overnight, with free access to water, for 12 h prior to the administration of the formulations. All pigs were in good health before each experiment, as determined by physical examination. On the morning of the experiment, pigs were sedated by a 10: 1 ketamine: xylazine solution, followed by maintenance of 2–3% isoflurane. Pigs received 5 mg/kg gentamycin antibiotic. 134 mg of each tested formulation, containing 0.75 mg hPTH(1–34) acetate (0.69 mg hPTH(1–34) as a free base), was delivered directly to the duodenum of sedated animals (Fig. 1) by a custom-made endoscope-based device, equipped with a chamber storing the formulation, a pneumatic mechanism for the release of the formulation from the chamber, and a live-video-camera to ensure repeatable delivery to the same location. In all animals, the granulate was released and clustered in an approximately the same location.
Fig. 1.
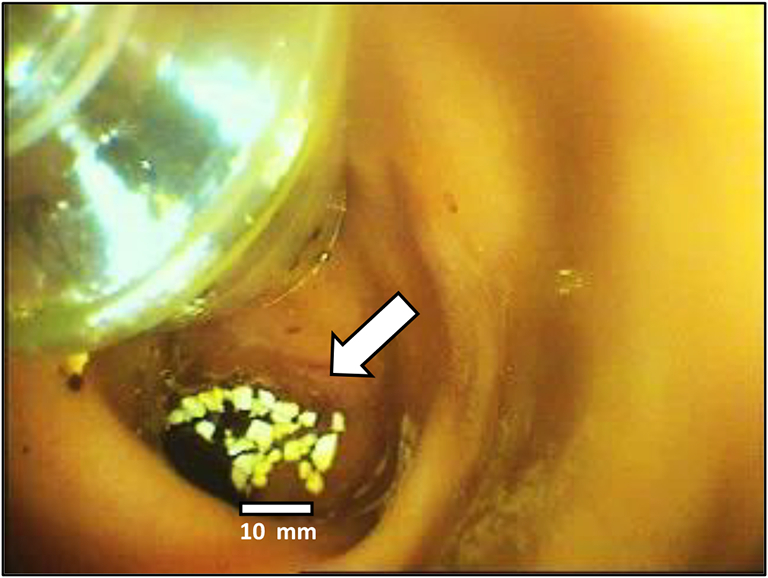
Delivery of the formulation in the form of a dry granulate (indicated by an arrow) to the duodenum by a custom-made endoscope-based device. The size bar shows the dimensions of the administered granules.
Sedation was terminated after approximately 30 min and pigs were enabled to wake up, while blood sampling continued. Blood samples of 3 mL were taken via an indwelling cannula in the right jugular vein into K3-EDTA vacutainers at the following time points: 10 min pre-dose, and after formulation administration at 5, 10, 15, 20, 30, 45, 60, 75, 90, 105, 120, and 150 min. A void of 1 mL was drawn and discarded before each sample, and the cannula was flushed with 1.5 mL normal saline after each sampling. Samples were kept on ice and centrifuged within 0.5 h at 2540 g, 4 °C for 10 min. Plasma was stored at −20 °C pending analysis.
2.8. Bioanalytical method
Quantification of plasma hPTH(1–34) was performed using a chemiluminescence-based immunoassay on the IDS-iSYS automated platform (Immunodiagnostic Systems, Boldon, UK), at the Bioanalytical Facility, University of East Anglia (UK) (Washbourne et al., 2012). Linearity was shown across the range of 4–100 pg/mL (R2 > 0.997). The lower limit of quantification (LLOQ), defined as the lowest quantifiable concentration at which the analytical coefficient of variation (CV) was less than 10%, was determined at 15 pg/mL. The intra-assay CV was 5.4% at concentration of 46.7 pg/mL and 7.0% at concentration of 11.7 pg/mL. Inter-assay CV was 7% at concentration of 45.1 pg/mL and 4.2% at concentration of 18.5 pg/mL.
2.9. Statistical and pharmacokinetic analysis
The area under the curve (AUC) of hPTH(1–34) concentration versus time was calculated by the linear trapezoidal rule separately for each pig. Statistical analysis of the pharmacokinetic (PK) parameters (Cmax and AUC) was performed by Kruskal-Wallis rank-sum test with Dunn post-hoc analysis, further adjusted by the Benjamini-Hochberg FDR method. Statistical significance was stated for p ≤ 0.05.
3. Results
3.1. In vitro evaluation of hPTH(1–34) protection by SBTI against enzymatic degradation
Table 2 shows in vitro results of SBTI inhibition of hPTH(1–34) proteolysis by trypsin and chymotrypsin. hPTH(1–34) alone was shown to be stable for at least one hour. When exposed to proteolytic enzymes trypsin and chymotrypsin hPTH(1–34) is rapidly degraded, reaching undetectable levels by two minutes, the first time point tested. However, in presence of SBTI enzymatic proteolysis was substantially inhibited. Following exposure to trypsin, 92% of the initial hPTH(1–34) concentration remained at 15 min (Tmax of the in vivo study) and 77% after an hour of incubation. Chymotrypsin was less susceptible to SBTI inhibition. Nevertheless, SBTI had still a substantial protective effect, with 59% of hPTH(1–34) remaining intact after 15 min.
Table 2.
In vitro study: inhibition by the soybean trypsin inhibitor of teriparatide's degradation by trypsin and chymotrypsin.
| Experiment | Molar ratio (mol/mol) protease: hPTH(1–34): SBTI | Percent of remaining intact hPTH(1–34) |
|||||
|---|---|---|---|---|---|---|---|
| 2 min | 5 min | 10 min | 15 min | 30 min | 60 min | ||
| hPTH(1–34) | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | |
| hPTH(1–34): trypsin | 11.4: 1.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| hPTH(1–34): chymotrypsin | 6.9: 1.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| hPTH(1–34): trypsin: SBTI | 11.4: 1.0: 149.4 | 94 ± 2 | 94 ± 2 | 94 ± 2 | 92 ± 1 | 86 ± 0 | 77 ± 1 |
| hPTH(1–34): chymotrypsin: SBTI | 6.9: 1.0: 90.2 | 78 ± 1 | 74 ± 0 | 67 ± 0 | 59 ± 0 | 39 ± 0 | 13 ± 1 |
Mean (±SD); n = 2. hPTH(1–34) – teriparatide (as acetate), SBTI – soybean trypsin inhibitor.
3.2. Dissolution test
The in vitro dissolution of the formulations was very rapid (Table 3). 100% of hPTH(1–34) was released within 5 min after the beginning of the test from the SNAC/SBTI/hPTH(1–34) and SNAC/hPTH(1–34) formulations. The SBTI/hPTH(1–34) formulation had a slightly slower dissolution rate. Nevertheless, a major part of the SBTI/hPTH(1–34) formulation's dose (72.6%) was released at the 5 min time point.
Table 3.
Percent of teriparatide acetate released from the granulate formulations in dissolution test.
| Time (min) | SNAC/SBTI/hPTH(1–34) | SNAC/hPTH(1–34) | SBTI/hPTH(1–34) |
|---|---|---|---|
| 0 | 0.0 (±0.0) | 0.0 (±0.0) | 0.0 (±0.0) |
| 5 | 100.0 (±3.6) | 100.0 (±0.0) | 72.6 (±8.1) |
| 10 | 100.0 (±3.6) | 100.0 (±0.0) | 93.5 (±0.0) |
| 15 | 100.0 (±3.6) | 100.0 (±0.0) | 100.0 (±6.5) |
| 20 | 100.0 (±3.6) | 100.0 (±0.0) | 100.0 (±0.0) |
Mean (±SD), n = 2; SNAC – salcaprozate sodium, SBTI – soybean trypsin inhibitor, hPTH(1–34) – teriparatide (as acetate).
3.3. In vivo study in pigs
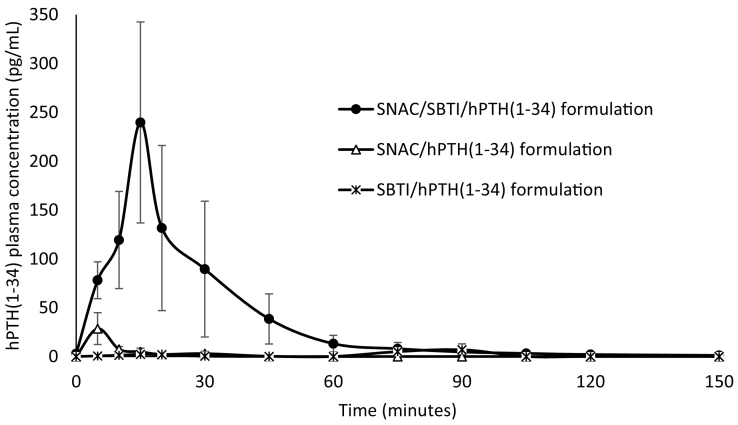
The PK profile of hPTH(1–34) following the administration of the three tested formulations is presented in Fig. 2, with the main PK parameters described in Table 4. The formulation containing both SNAC and SBTI (SNAC/SBTI/hPTH(1–34) formulation) was shown, with statistical significance, to enable higher plasma exposure of hPTH(1–34) compared to the other two formulations (~10- and ~20-fold higher Cmax and AUC, respectively, compared to both the other two formulations). No statistically significant difference was found between the SNAC/hPTH(1–34) and SBTI/hPTH(1–34) formulations, both enabling only slight increase in plasma concentrations over baseline.
Fig. 2.
Teriparatide plasma concentration vs. time plot (mean ± SEM) following administration of formulations in the form of a dry granulate containing 0.75 mg teriparatide acetate (0.69 mg teriparatide as a free base) directly to the duodenum of female pigs by a custom-made endoscope-based device; SNAC – salcaprozate sodium, SBTI – soybean trypsin inhibitor, hPTH(1–34) – teriparatide (as acetate); SNAC/SBTI/hPTH(1–34) formulation: n = 5; SNAC/hPTH(1–34) formulation: n = 5; SBTI/hPTH(1–34) formulation: n = 4.
Table 4.
Mean (±SEM) pharmacokinetic parameters obtained following administration by a custom-made endoscope-based device of dry granulate formulations containing 0.75 mg teriparatide acetate (0.69 mg teriparatide as a free base) directly to the duodenum of female pigs.
| Formulation ID | No. of animals | Cmax (pg/mL) | Tmaxb (min) | AUC0-last (min*pg/mL) |
|---|---|---|---|---|
| SNAC/SBTI/hPTH(1–34) | 5 | 259.6a (±96.2) | 5 (5–15) | 5310.8a (±2745.8) |
| SNAC/hPTH(1–34) | 5 | 28.6 (±16.3) | 5 (5) | 262.4 (±157.1) |
| SBTI/hPTH(1–34) | 4 | 9.6 (±5.3) | 25 (15–90) | 218.5 (±158.0) |
SNAC – salcaprozate sodium, SBTI – soybean trypsin inhibitor, hPTH(1–34) – teriparatide (as acetate), Cmax – maximal plasma concentration, Tmax – time to reach maximal plasma concentration, AUC0-last – area under the curve calculated to the last sampling time point.
Differs with statistical significance from the other two groups with p ≤ 0.05 (Kruskal-Wallis rank-sum test with Dunn post-hoc analysis, further adjusted by the Benjamini-Hochberg FDR method).
Tmax presented as a median with range.
4. Discussion
The vast majority of biological drugs require parenteral administration due to their inherent chemical instability throughout the GIT and negligible permeability across the intestinal epithelial lining. In recent years, a concerted effort has been made to overcome these absorption challenges and create an oral dosage form of these drugs. Two approaches stand out because of their advanced stages of development. One approach is based on the enhancement of the drug's permeability through the epithelial membrane and the second is based on the prevention of proteolysis. Each of these individual approaches is capable of augmenting systemic exposure of orally delivered peptides and proteins, albeit to a limited extent. In the current work, we studied the two methods each on their own, and in combination in vivo in pigs. To the best of our knowledge, this is the first report of in vivo evaluation of the combination of these two delivery methods utilized for the oral delivery of macromolecules.
An in-depth investigation undertaken by Novo Nordisk A/S on the permeation enhancement action of SNAC showed the stomach as the main absorption site for semaglutide (Rybelsus) (Buckley et al., 2018). In our current work, we showed in vivo by an example of hPTH(1–34) that a peptide drug appropriately co-formulated with SNAC can be also effectively absorbed from the small intestine.
Results of the current study showed that hPTH(1–34) delivered only with a protease inhibitor (SBTI/hPTH(1–34) formulation), although protected from proteolytic degradation, cannot effectively permeate the lipophilic membranes of the epithelial cells due to hydrophilic nature and large size. Consequently, its plasma concentrations were almost undetectable. When the permeation enhancer SNAC is present without the protease inhibitor in the formulation (SNAC/hPTH(1–34) formulation) an insufficient amount of the intact drug is present in the intestinal lumen to be absorbed, as it is rapidly degraded by the intestinal proteolytic enzymes and thus, only very low plasma concentrations of hPTH(1–34) were detected. When these two approaches are appropriately combined, higher systemic absorption of the peptide can be achieved.
As hPTH(1–34) is highly sensitive to proteolysis by the intestinal enzymes trypsin and chymotrypsin (Werle et al., 2006), it was co-formulated with a protease inhibitor (SBTI). To evaluate the ability of SBTI to protect hPTH(1–34) from proteolysis, an in vitro study was undertaken prior to the study in pigs. Results of the study showed that SBTI was highly potent in protecting hPTH(1–34) from proteolysis by trypsin; inhibition of chymotrypsin activity was limited but still significant. As SNAC's effect on membrane permeability is relatively short (Buckley et al., 2018), leading to a Tmax of 5–15 min (Table 4), luminal proteolysis inhibition by SBTI is effective in preserving a major portion of the delivered dose of the drug intact.
As the dissolution rate may potentially impact the local concentrations of the permeation enhancer, which is critical for effective enhancement of the drug's absorption (as previously discussed), a comparative dissolution test of the three formulations was performed (Table 3). For the three tested formulations the dissolution was very fast. Therefore, this factor was not included in the analysis of the data; in practical terms, it may be considered as if the constituents of the three formulations were released at the application site rapidly and simultaneously. Consequently, it can be deduced that the dissolution rate is not the reason for the different PK profiles of hPTH(1–34) obtained in the three tested groups in vivo.
Implications of the current study are not limited only to the constituents of the tested formulation. We believe that many attempts of per oral delivery of macromolecules undertaken in the past were unsuccessful due, at least partially, to a limited understanding of GIT physiological mechanisms, resulting in a suboptimal pharmaceutical delivery method. The appropriate combination of several absorption enhancing approaches such as those suggested here will enable more effective oral delivery of these drugs.
The purpose of the current work was to study the mechanisms of oral delivery of biological drugs. Usually, mechanistic studies are performed in in vitro and ex vivo systems, representing an isolated tissue or organ of interest, however, the entire biologic system of the intact body, including the variety of parameters affecting absorption, is much more complex (Dahan et al., 2012; Wilding, 2000). Direct delivery to the duodenum in the current in vivo study was performed in order to isolate the effect of the formulation excipients on the absorption of hPTH(1–34), preventing the influence of stomach-related physiological processes: gastric emptying time, pH, stomach motility, etc. These parameters may potentially bias the results and increase their variability, making the interpretation of the data difficult. Yet, when directly administering the formulation to the area of interest in vivo, only the relevant physiological processes are present, simplifying the understanding of the contribution of the formulation components to the absorption process of the studied drug. In current work, the variability in the results was still high, probably rising from a known variability of absorption of peptide and protein drugs in the GIT (Tyagi et al., 2018). Still, the results were found to be statistically significant, enabling rational conclusions for the studied objectives to be drawn.
Regarding the molecule studied in this work – hPTH(1–34), the PK profile of the oral formulation obtained in the current study is of particular interest. While the bioavailability of hPTH(1–34) is important for bone homeostasis, the PK profile of hPTH(1–34) is also critical. It is well established that intermittent administration of pharmacological doses of hPTH(1–34) has an anabolic effect on the bone, while continuous hPTH administration is detrimental for the skeleton due to stimulation of bone resorption (Hock and Gera, 1992). These PK profiles suggest that the anabolic or catabolic response of bone to hPTH(1–34) is determined primarily by the length of time that serum concentrations of hPTH(1–34) remain above baseline levels of endogenous PTH and only secondarily by the Cmax or AUC of hPTH(1–34) achieved (Frolik et al., 2003). The optimal PK profile for an anabolic effect and therapeutic response of hPTH(1–34) is characterized by rapid absorption and rapid elimination (Satterwhite et al., 2010), which were also observed in the current study with hPTH(1–34) delivered to intestine with the combination of the two employed methods. This profile was comparable to the PK profile of a subcutaneous injection (Forteo) utilized for the treatment of osteoporotic women (Satterwhite et al., 2010). These promising results paved the way for the clinical development of an oral SNAC-SBTI combination-based hPTH(1–34) formulation for the treatment of osteoporosis.
5. Conclusions
The current study highlights the potential of an appropriate combination of the two oral drug delivery approaches – permeation enhancement and proteolysis inhibition, to effectively increase plasma exposure of peptide and protein drugs following peroral administration. This combination methodology is yet to be fully exploited and could be further implemented for more effective oral delivery of a variety of therapeutic biomacromolecules.
The following are the supplementary data related to this article.
HPLC chromatogram following separation on a ZORBAX 300SB C-18, 4.6*150 mm column with particle size 3.5 μm protected by a guard column maintained at temperature of 40 oC. Elution of hPTH(1-34) at ~8 min was obtained with flow of 1.0 mL/min of mobile phase comprised of a 1:3 mixture of acetonitrile : 0.2 M sulfate buffer pH 2.3. Detection was performed at 214 nm. Trypsin, chemotrypsin, and soybean trypsin inhibitor elute at ~2 min. The work was performed on the HPLC system Agilent 1100 series.
Disclosures
GB, CI, HG, and PS are employees of Entera Bio Ltd. JCYT and WDF received financial support from Entera Bio Ltd. to perform the analysis.
Role of the funding source
This research was designed, conducted, and financially supported by Entera Bio Ltd. The manuscript was prepared by Entera Bio Ltd.
Declaration of Competing Interest
None.
Acknowledgements
Ehud Arbit MD and Lital Friedman are highly acknowledged for their constructive comments and assistance in preparation of the manuscript. Eli Reichman and Ariel Rothner are acknowledged for their valuable technical assistance. The in vivo study was performed at Biotech Farm Ltd. (Mazkeret Batya, Israel) by its veterinary staff. This research was financially supported by Entera Bio Ltd.
References
- Aguirre T.A., Teijeiro-Osorio D., Rosa M., Coulter I.S., Alonso M.J., Brayden D.J. Current status of selected oral peptide technologies in advanced preclinical development and in clinical trials. Adv. Drug Deliv. Rev. 2016;106(Pt B):223–241. doi: 10.1016/j.addr.2016.02.004. https://www.ncbi.nlm.nih.gov/pubmed/26921819 [DOI] [PubMed] [Google Scholar]
- Alani A.W., Robinson J.R. Mechanistic understanding of oral drug absorption enhancement of cromolyn sodium by an amino acid derivative. Pharm. Res. 2008;25(1):48–54. doi: 10.1007/s11095-007-9438-6. https://www.ncbi.nlm.nih.gov/pubmed/17846867 [DOI] [PubMed] [Google Scholar]
- Animals, National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory . 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Birk Y. Trypsin and chymotrypsin inhibitors from soybeans. Methods Enzymol. 1976;45:700–707. doi: 10.1016/s0076-6879(76)45061-9. https://www.ncbi.nlm.nih.gov/pubmed/1034864 [DOI] [PubMed] [Google Scholar]
- Brayden D., Creed E., O’Connell A., Leipold H., Agarwal R., Leone-Bay A. Heparin absorption across the intestine: effects of sodium N-[8-(2-hydroxybenzoyl)amino]caprylate in rat in situ intestinal instillations and in Caco-2 monolayers. Pharm. Res. 1997;14(12):1772–1779. doi: 10.1023/a:1012192115828. https://www.ncbi.nlm.nih.gov/pubmed/9453067 [DOI] [PubMed] [Google Scholar]
- Brayden D.J., Hill T.A., Fairlie D.P., Maher S., Mrsny R.J. Systemic delivery of peptides by the oral route: Formulation and medicinal chemistry approaches. Adv. Drug Deliv. Rev. 2020;157:2–36. doi: 10.1016/j.addr.2020.05.007. https://www.ncbi.nlm.nih.gov/pubmed/32479930 [DOI] [PubMed] [Google Scholar]
- Bucheit J.D., Pamulapati L.G., Carter N., Malloy K., Dixon D.L., Sisson E.M. Oral semaglutide: a review of the first oral glucagon-like peptide 1 receptor agonist. Diabetes Technol. Ther. 2020;22(1):10–18. doi: 10.1089/dia.2019.0185. https://www.ncbi.nlm.nih.gov/pubmed/31436480 [DOI] [PubMed] [Google Scholar]
- Buckley S.T., Bækdal T.A., Vegge A., Maarbjerg S.J., Pyke C., Ahnfelt-Rønne J., Madsen K.G., Schéele S.G., Alanentalo T., Kirk R.K., Pedersen B.L., Skyggebjerg R.B., Benie A.J., Strauss H.M., Wahlund P.O., Bjerregaard S., Farkas E., Fekete C., Søndergaard F.L., Borregaard J., Hartoft-Nielsen M.L., Knudsen L.B. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci. Transl. Med. 2018;10(467) doi: 10.1126/scitranslmed.aar7047. https://www.ncbi.nlm.nih.gov/pubmed/30429357 [DOI] [PubMed] [Google Scholar]
- Burshtein G., Tang J.C.Y., Rothner A., Galitzer H., Schwartz P., Fraser W. ASBMR 2017 Annual Meeting. 2017. Enhanced bioavailability and reduced pharmacokinetic variability of Oral PTH (1–34) in man. [Google Scholar]
- Burshtein, G, A Rothner, P Schwartz, and H Galitzer. 2018. Treatment of hypoparathyroidism. U.S. Patent Publication number US20180050096.
- Burshtein, G., A., Rothner, P Schwartz, and H Galitzer. 2018b. Treatment of osteoporosis. U.S. Patent Publication number US20180028622.
- Dahan A., Lennernäs H., Amidon G.L. The fraction dose absorbed, in humans, and high jejunal human permeability relationship. Mol. Pharm. 2012;9(6):1847–1851. doi: 10.1021/mp300140h. https://www.ncbi.nlm.nih.gov/pubmed/22524707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldor R., Kidron M., Arbit E. Open-label study to assess the safety and pharmacodynamics of five oral insulin formulations in healthy subjects. Diabetes Obes. Metab. 2010;12(3):219–223. doi: 10.1111/j.1463-1326.2009.01153.x. https://www.ncbi.nlm.nih.gov/pubmed/20151998 [DOI] [PubMed] [Google Scholar]
- Eldor R., Arbit E., Corcos A., Kidron M. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLoS One. 2013;8(4):e59524. doi: 10.1371/journal.pone.0059524. https://www.ncbi.nlm.nih.gov/pubmed/23593142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosgerau K., Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov. Today. 2015;20(1):122–128. doi: 10.1016/j.drudis.2014.10.003. https://www.ncbi.nlm.nih.gov/pubmed/25450771 [DOI] [PubMed] [Google Scholar]
- Frolik C.A., Black E.C., Cain R.L., Satterwhite J.H., Brown-Augsburger P.L., Sato M., Hock J.M. Anabolic and catabolic bone effects of human parathyroid hormone (1-34) are predicted by duration of hormone exposure. Bone. 2003;33(3):372–379. doi: 10.1016/s8756-3282(03)00202-3. https://www.ncbi.nlm.nih.gov/pubmed/13678779 [DOI] [PubMed] [Google Scholar]
- Hernández-Ledesma B., Hsieh C.C. Chemopreventive role of food-derived proteins and peptides: a review. Crit. Rev. Food Sci. Nutr. 2017;57(11):2358–2376. doi: 10.1080/10408398.2015.1057632. https://www.ncbi.nlm.nih.gov/pubmed/26565142 [DOI] [PubMed] [Google Scholar]
- Hess S., Rotshild V., Hoffman A. Investigation of the enhancing mechanism of sodium N-[8-(2-hydroxybenzoyl)amino]caprylate effect on the intestinal permeability of polar molecules utilizing a voltage clamp method. Eur. J. Pharm. Sci. 2005;25(2–3):307–312. doi: 10.1016/j.ejps.2005.03.003. https://www.ncbi.nlm.nih.gov/pubmed/15911227 [DOI] [PubMed] [Google Scholar]
- Hock J.M., Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J. Bone Miner. Res. 1992;7(1):65–72. doi: 10.1002/jbmr.5650070110. https://www.ncbi.nlm.nih.gov/pubmed/1532281 [DOI] [PubMed] [Google Scholar]
- Ish-Shalom S., Caraco Y., Khazen N.S., Gershinsky M., Szalat A., Schwartz P., Arbit E., Galitzer H., Tang J.C., Burshtein G., Rothner A., Raskin A., Blum M., Fraser W.D. Safety and efficacy of oral human parathyroid hormone (1-34) in hypoparathyroidism: an open-label study. J. Bone Miner. Res. 2021;36(6):1060–1068. doi: 10.1002/jbmr.4274. https://www.ncbi.nlm.nih.gov/pubmed/33666947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdal M.A., Riis B.J., Mehta N., Stern W., Arbit E., Christiansen C., Henriksen K. Lessons learned from the clinical development of oral peptides. Br. J. Clin. Pharmacol. 2015;79(5):720–732. doi: 10.1111/bcp.12557. https://www.ncbi.nlm.nih.gov/pubmed/25408230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M., Kato I. Protein inhibitors of proteinases. Annu. Rev. Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. https://www.ncbi.nlm.nih.gov/pubmed/6996568 [DOI] [PubMed] [Google Scholar]
- Lennernäs H., Abrahamsson B., Persson E.M., Knutson L. Oral drug absorption and the biopharmaceutics classification system. J. Drug Del. Sci. Tech. 2007;17(4):237–244. [Google Scholar]
- Leone-Bay A., Sato M., Paton D., Hunt A.H., Sarubbi D., Carozza M., Chou J., McDonough J., Baughman R.A. Oral delivery of biologically active parathyroid hormone. Pharm. Res. 2001;18(7):964–970. doi: 10.1023/a:1010936227570. https://www.ncbi.nlm.nih.gov/pubmed/11496956 [DOI] [PubMed] [Google Scholar]
- Malkov D., Wang H.Z., Dinh S., Gomez-Orellana I. Pathway of oral absorption of heparin with sodium N-[8-(2-hydroxybenzoyl)amino] caprylate. Pharm. Res. 2002;19(8):1180–1184. doi: 10.1023/a:1019802310702. https://www.ncbi.nlm.nih.gov/pubmed/12240944 [DOI] [PubMed] [Google Scholar]
- McIntyre C., Schmidt J., Castelli M.C., Bittner B. Study on the impact of SNAC (sodium N-[8-(2-hydroxybenzoyl) amino] caprylate) on the bioavailability of ibandronate (IBN) in postmenopausal women. J. Drug Deliv. Sci. Technol. 2011;21(6):521–525. (doi:doi.org/10.1016/S1773-2247(11)50084-X) [Google Scholar]
- Neer R.M., Arnaud C.D., Zanchetta J.R., Prince R., Gaich G.A., Reginster J.Y., Hodsman A.B., Eriksen E.F., Ish-Shalom S., Genant H.K., Wang O., Mitlak B.H. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001;344(19):1434–1441. doi: 10.1056/NEJM200105103441904. https://www.ncbi.nlm.nih.gov/pubmed/11346808 [DOI] [PubMed] [Google Scholar]
- Satterwhite J., Heathman M., Miller P.D., Marín F., Glass E.V., Dobnig H. Pharmacokinetics of teriparatide (rhPTH[1-34]) and calcium pharmacodynamics in postmenopausal women with osteoporosis. Calcif. Tissue Int. 2010;87(6):485–492. doi: 10.1007/s00223-010-9424-6. https://www.ncbi.nlm.nih.gov/pubmed/20953593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart A.L., Gaisford S., Basit A.W. Oral peptide and protein delivery: intestinal obstacles and commercial prospects. Expert. Opin. Drug Deliv. 2014;11(8):1323–1335. doi: 10.1517/17425247.2014.917077. https://www.ncbi.nlm.nih.gov/pubmed/24816134 [DOI] [PubMed] [Google Scholar]
- Twarog C., Fattah S., Heade J., Maher S., Fattal E., Brayden D.J. Intestinal permeation enhancers for oral delivery of macromolecules: a comparison between salcaprozate sodium (SNAC) and Sodium Caprate (C) Pharmaceutics. 2019;11(2) doi: 10.3390/pharmaceutics11020078. https://www.ncbi.nlm.nih.gov/pubmed/30781867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twarog C., Liu K., O’Brien P.J., Dawson K.A., Fattal E., Illel B., Brayden D.J. A head-to-head Caco-2 assay comparison of the mechanisms of action of the intestinal permeation enhancers: SNAC and sodium caprate (C) Eur. J. Pharm. Biopharm. 2020;152:95–107. doi: 10.1016/j.ejpb.2020.04.023. https://www.ncbi.nlm.nih.gov/pubmed/32387703 [DOI] [PubMed] [Google Scholar]
- Tyagi P., Pechenov S., Subramony J.A. Oral peptide delivery: Translational challenges due to physiological effects. J. Control. Release. 2018;287:167–176. doi: 10.1016/j.jconrel.2018.08.032. https://www.ncbi.nlm.nih.gov/pubmed/30145135 [DOI] [PubMed] [Google Scholar]
- Washbourne C., Tang J., Fraser W. SU0120. The effects of storage temperature and repeat freeze-thaw cycles on stability of PTH(1-34) as determined by the IDS-iSYS Automated Analyser. J. Bone Miner. Res. 2012;27(S1):S250. doi: 10.1002/jbmr.1852. doi: 10.1002/jbmr.1852. [DOI] [Google Scholar]
- Welling S.H., Hubálek F., Jacobsen J., Brayden D.J., Rahbek U.L., Buckley S.T. The role of citric acid in oral peptide and protein formulations: relationship between calcium chelation and proteolysis inhibition. Eur. J. Pharm. Biopharm. 2014;86(3):544–551. doi: 10.1016/j.ejpb.2013.12.017. https://www.ncbi.nlm.nih.gov/pubmed/24384069 [DOI] [PubMed] [Google Scholar]
- Werle M., Samhaber A., Bernkop-Schnürch A. Degradation of teriparatide by gastro-intestinal proteolytic enzymes. J. Drug Target. 2006;14(3):109–115. doi: 10.1080/10611860600647934. https://www.ncbi.nlm.nih.gov/pubmed/16753824 [DOI] [PubMed] [Google Scholar]
- Wilding I. Site-specific drug delivery in the gastrointestinal tract. Crit. Rev. Ther. Drug Carrier Syst. 2000;17(6):557–620. https://www.ncbi.nlm.nih.gov/pubmed/11204736 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HPLC chromatogram following separation on a ZORBAX 300SB C-18, 4.6*150 mm column with particle size 3.5 μm protected by a guard column maintained at temperature of 40 oC. Elution of hPTH(1-34) at ~8 min was obtained with flow of 1.0 mL/min of mobile phase comprised of a 1:3 mixture of acetonitrile : 0.2 M sulfate buffer pH 2.3. Detection was performed at 214 nm. Trypsin, chemotrypsin, and soybean trypsin inhibitor elute at ~2 min. The work was performed on the HPLC system Agilent 1100 series.