Abstract
Objectives
Metabolic syndrome (MetS) is the constellation of cardio-metabolic risk factors, and it can illustrate the coming burden of cardiovascular diseases and diabetes mellitus. Myanmar faces larger pressure from cardio-metabolic diseases and there is no existing data to understand the magnitude of MetS in adult population. This study aimed to investigate the extent of existing people with MetS in the community and to know the related lifestyle factors to MetS.
Methods
A community based cross-sectional study was performed in convenient sample of 302 Myanmar adult people. Prevalence of MetS and associated risk factors were identified by collecting sociodemographic information, anthropometric measurements, and blood investigation of glucose and lipid profiles. Logistic regression analysis was performed for developing statistical models to estimate the odd ratios (OR) and confidence intervals (CI).
Results
Prevalence of MetS is 29.1%, and there is no significant difference between male and female prevalence. Abdominal obesity is the highest component of MetS (OR: 28.37, CI: 12.41–64.85, p < 0.001) especially for female (OR: 44.52, CI: 17.19–115.31, p < 0.001), and hypertriglyceridemia (OR: 11.05, CI: 6.20–19.66, p < 0.001) is the commonest in male. Age is the constant risk for developing MetS (OR: 4.06, CI: 1.91–8.64, p < 0.001) whereas the practice of midday nap is the behavior related to increased occurrence of MetS (OR: 1.97, CI: 1.16–3.38, p < 0.05). Dietary pattern, smoking status, drinking alcoholic beverages and physical activity involvement do not impact on the development of MetS. Education, occupation, income and other sociodemographic factors do not produce significant effect on cardio-metabolic status. Obesity, diabetes, and hypertensive conditions of community residents are also explored.
Conclusions
Modifiable pathophysiological conditions such as abdominal obesity, and obesity play a vital role in preventing MetS before it occurs while unmodifiable risk factor such as getting older and female sex are not as much important as changeable lifestyle habits.
Keywords: Adult people, Cross-sectional study, Lifestyles, Metabolic syndrome, Prevalence, Risk factors
Highlights
-
•
Prevalence of Metabolic Syndrome in Myanmar adult people is 29.1%.
-
•
Abdominal obesity is the commonest component.
-
•
Aging, obesity, diabetes, and hypertensive conditions are risk factors.
-
•
Practice of midday nap is a potential risky behavior.
-
•
Myanmar needs more attention to public health.
1. Introduction
Metabolic syndrome (MetS) is the condition of the existence of multiple metabolic risk factors for cardiovascular diseases (CVD) and diabetes mellitus (DM). The risk factors involve increased blood pressure (BP), dyslipidemia (higher triglycerides (TG) and lowered high-density lipoprotein (HDL) cholesterol), increased fasting blood sugar (FBS), and central obesity. Over a billion people around the world are now affected with MetS [1]. Individual with MetS has the double chance of occurring non-communicable diseases (NCD) such as CVD over the next five to 10 years than the one without MetS. It is also responsible for the 5-fold increase in risk for type 2 DM [2]. The financial difficulty from medical cost could reach two to three times higher for people with MetS [3]. Many scientific studies explored the causal factors that lead to MetS which include smoking, sedentary lifestyle, unhealthy diet, and lack of physical exercises. In some countries with mature health system, chronic diseases are being prevented by early identification and promoting healthy lifestyles. Comprehensive health care services for targeted age group and population-based health survey establish databases on population health, behavior, lifestyle, and nutrition. Analyzing and inferencing such existing meta-data provoke much evidence-based policy making for health promotion and disease prevention [4].
Worldwide, the prevalence of MetS ranges from 20% to 60% in multiple ethnic backgrounds [5]. Even in developed countries, smoking cessation, glucose control, obesity prevention and maintaining normal BP are still challenging for primary care services [6,7]. Sociodemographic factors such as age, sex, family history, and economic status are also related to increasing trend of MetS. In certain Asian countries, lower secondary education, people in urban area, and unstable body weight since one's twenties are additional risk factors to escalation of MetS [[8], [9], [10]]. In Myanmar, mortality from CVD reached 25% of all death and DM accounted for 4% [11]. Absence of nation-wide health surveys, community-based health check-up program, and national health insurance system leads to nonexistence of health-oriented population-based data and unavailability of reliable evidence to fight against NCD before it occurs. Despite there are some epidemiological studies emphasize on metabolic status of children and adolescents [12,13], findings for adult population are still limited. Scientific evidence for lifestyle characteristics is inadequate and the impact of those daily habits on risk factors of MetS is undiscovered. Therefore, this study aimed to identify the prevalence of MetS and its components in Myanmar adult people, and to investigate the association between lifestyle factors and risk factors of MetS.
2. Materials and methods
A community based cross-sectional study was performed in a municipal township with high occurrence of NCD in sub-urban area of Yangon region, Myanmar. Data collection by home visits took five days in November 2020 by applying Covid-19 preventive measures for researcher, data collectors and participants. Five registered nurses with specialized knowledge in community health were trained and rechecked to prevent observer bias for collecting sociodemographic, lifestyle data and anthropometric measurements in the community. Blood sample for lipid profiles, and blood glucose were gathered by three phlebotomists. Adult over 18 years old who are living in study area for at least one year without pregnancy conditions, and medical problems such as cardiac diseases, diabetes, and renal disorders were recruited by convenience sampling method from three municipal wards of selected township. People who are taking anti-diabetic, anti-hypertensive, and lipid-lowering agents, and who cannot involve in anthropometric measurements were excluded from this study. Tentative participants who showed their interest in this study were fully explained about the purpose of the study, body size measurements, and invasive blood withdrawing process. Informed written consent was attained from all individuals who are available both for physical examinations and blood investigations.
Sociodemographic data included age, sex, marital status, ethnics and religion, education, occupation, income, length of living in current place, numbers of family members and numbers of children. Bodyweight, height, waist circumference (WC), and BP were measured according to the guidelines of WHO STEP survey by using digital weight scale in kilogram (kg), portable mechanical stadiometer (cm), non-elastic tape measure (cm), and electronic BP monitor (Yuwell, YE660E) [14]. To measure bodyweight, digital scale was ensured to reach zero mark before the participant step on it. Removed footwear, heavy clothes, and extra items from the participants and requested them to step on the scale with both feet, to look straight ahead, and stay still until the digital numbers settled down. Weight was documented to the nearest kg. To measure height, participants were requested to take off their footwear and positioned them with their back to the measuring rod on the stadiometer, confirming their feet are together and facing forward and their heels are touching the heel plate. Unnecessary accessories on their head and extra hair designs were removed before measurement. Then, they were requested to look straight ahead and adjust the head plate until it touches the top of their head. Height was recorded to the nearest cm. To measure WC, non-stretchable tape measure was used at the midpoint between the iliac crest and the lower margin of the ribs. Participants were instructed to stand upright in their both feet and recorded the number on the tape measure right after they exhaled.
Body mass index (BMI) was calculated from bodyweight and height in kg/m2. All measurements for weight, height, WC and BP were taken twice and average value of those two assessments were documented to prevent measurement error. Lipid profiles such as total cholesterol, TG, HDL, low-density lipoprotein (LDL) and blood glucose investigations such as FBS and glycated hemoglobin (HbA1c) level were assayed by taking 5 mm of overnight fasting venous blood and analyzed by using COBAS C 111 analyzer, Roche Diagnostics Ltd., Switzerland.
Information on lifestyle characteristics such as sleeping habits, eating behavior, smoking and alcohol drinking, smokeless tobacco consumption, physical activity and sedentary conditions, practicing medical check-up, and family history of chronic diseases were collected by structured questionnaires with two categories of response (yes/no). Question items were developed based on WHO STEPS instruments for NCD risk factors and training of trainer manual for package of essential NCD interventions (PEN) [15,16]. Internal consistency of questionnaires was evaluated by Cronbach's alpha (over 0.8) and content validation was considered as excellent by five expert persons. Pilot testing of lifestyle questionnaires were performed by 10 adult people who were not involved in actual study. Assessment of sleeping habits included duration of sleep, adequacy of sleeping, and practice of midday nap. Eating behavior assessment included frequency of meal per day, practice of skipping breakfast or dinner, largest meal (breakfast, lunch, dinner), eating snack between meals, eating home-cooked meal, eating typical Myanmar meal, and eating meal quickly. Paying attention to eating healthy meal and the consumption of salty meal, oily meal, fruits and vegetables, fish, meat and eggs were also asked. Respondents needed to tick ‘yes’ if they practiced those behavior three days per week. The habit of drinking alcohol, caffeinated beverages, smoking cigarettes, and intake of smokeless tobacco were investigated based on the habit of last six months. The pattern of physical activity was evaluated by the practice of physical exercise, acknowledging the benefits of doing physical exercise, presence of work-related physical activity, presence of work-related sedentary lifestyle, practice of sedentary behavior during leisure time, practice of walking quickly (compared with another companion). Weight changes of respondents was estimated based on last 10 years bodyweight variation. The practice of doing health check-up and family history of NCD were also investigated.
Sample size was considered by the prevalence of 20% according to the finding from previous studies along with a 5% precision, and confidence interval of 95%. Since the total adult population of study township is about 300,000, the sample size required is 246 participants. By adding 25% for possible missing data, necessary total sample size is 302 participants. Statistical analyses were conducted by Statistical Package for the Social Sciences (SPSS) version 26 for Mac. Participants’ characteristics were classified by people with MetS and people without MetS. The status of MetS was decided by the criteria of the National Cholesterol Education Program Adult Treatment Panel III. If three or more of the following criteria are met, it was defined as MetS: WC over 40 inches (male) or 35 inches (female), BP over 130/85 mmHg, fasting TG level over 150 mg/dl, fasting HDL cholesterol level less than 40 mg/dl (male) or 50 mg/dl (female) and FBS over 100 mg/dl [17]. Regarding WC, Asian cutoff points were utilized instead of aforementioned definition. It means WC is equivalent to or over 90 cm for male and 80 cm for female [18]. The difference of characteristics between two groups was compared by independent-samples t-test and chi-square. Variables that are significantly different were furthered assessed by logistic regression to identify the relationship with MetS. Statistical models were developed to show the strength of relationship with MetS (odd ratio (OR) and related confidence interval (CI)). A p-value <0.05 was supposed as statistically significant. Reporting of the study result followed the STROBE statement (Appendix). This study was approved by the Ethics and Research Committee of the University of Nursing, Yangon, Myanmar (ID: 32/2020).
3. Results
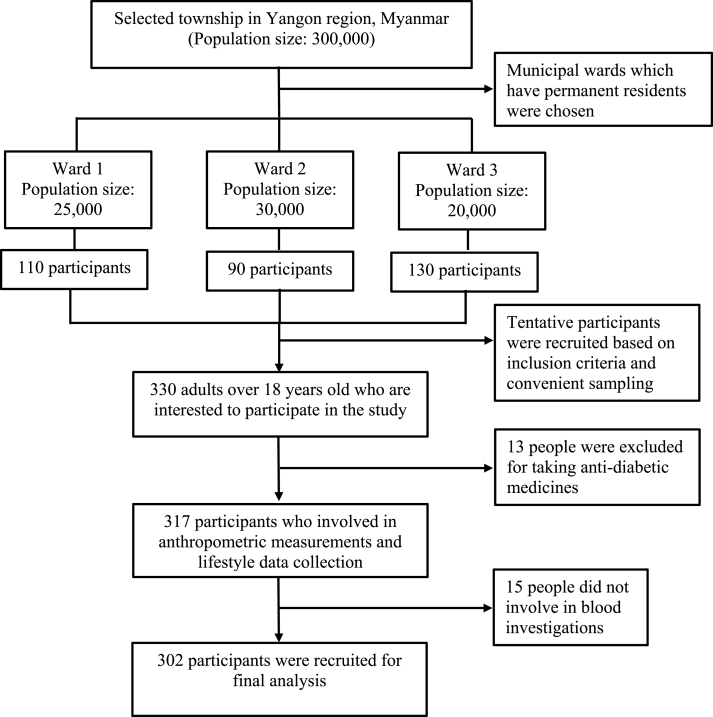
Initially there were 330 people who showed their interest in the study. Among them, 13 people were excluded for taking anti-diabetic medications, and 15 people did not involve in taking blood samples. Finally, 302 participants (210 female) participated in final analysis (Fig. 1). Prevalence of MetS was 29.1% (27.2% for male and 30.0% for female, p = 0.619). Sociodemographic, pathophysiological and lifestyle characteristics of participants were summarized based on presence or absence of MetS (Table 1). People with MetS has significantly increased in age, duration of living in current area, number of children, the practice of midday nap, acknowledging the benefits of physical exercise, getting weight within last 10 years, and the practice of health examination. Apart from those characteristics, the habit of dietary intake, alcohol drinking, smokeless tobacco consumption, physical activity, sedentary lifestyle, and family history of NCD were not different between people with and without MetS.
Fig. 1.
Flow chart for participant selection.
Table 1.
Socio-demographic, pathophysiological and lifestyle characteristics of participants based on status of MetS (n = 302).
| Variables | Presence of MetS (n = 88) |
Absence of MetS (n = 214) |
P-value | ||
|---|---|---|---|---|---|
| Mean (SD) | Frequency (%) | Mean (SD) | Frequency (%) | ||
| Age (years) | 48.03 (13.67) | 41.53 (14.52) | 0.000 | ||
| Gender | 0.619 | ||||
| Male | 25 (28.4) | 67 (31.3) | |||
| Female | 63 (71.6) | 147 (68.7) | |||
| Race | 0.538 | ||||
| Bamar | 87 (98.9) | 210 (98.1) | |||
| India | 1 (1.1) | 1 (0.5) | |||
| Rakhine | 0 (0) | 1 (0.5) | |||
| Pa Laung | 0 (0) | 1 (0.5) | |||
| Ka Mel | 0 (0) | 1 (0.5) | |||
| Religion | |||||
| Buddhist | 88 (100) | 212 (99.1) | |||
| Islam | 0 (0) | 2 (0.9) | |||
| Marital status | 0.648 | ||||
| Never married | 8 (9.1) | 31 (14.5) | |||
| Married | 65 (73.9) | 142 (66.4) | |||
| Divorced | 4 (4.5) | 12 (5.6) | |||
| Widow/er | 11 (12.5) | 28 (13.1) | |||
| Unwillingness to answer | 0 (0) | 1 (0.5) | |||
| Years of education | 5.82 (3.83) | 6.07 (3.73) | 0.597 | ||
| Occupation | 0.056 | ||||
| Government employee (0) | 0 (0) | 5 (2.3) | |||
| Private employee (1) | 6 (6.8) | 27 (12.6) | |||
| Laborer (2) | 2 (2.3) | 19 (8.9) | |||
| Private business (3) | 32 (36.4) | 74 (34.6) | |||
| Students (4) | 0 (0) | 6 (2.8) | |||
| Retired (5) | 1 (1.1) | 1 (0.5) | |||
| No income works (6) | 0 (0) | 1 (0.5) | |||
| Dependence (7) | 2 (2.3) | 1 (0.5) | |||
| Unemployment (able) (8) | 37 (42.0) | 69 (32.2) | |||
| Unemployment (unable) (9) | 8 (9.1) | 11 (5.1) | |||
| Income per month (Kyats) | 138295.97 (264866.74) | 116317.76 (129350.46) | 0.334 | ||
| Living years in current area (years) | 31.55 (20.33) | 26.19 (17.27) | 0.021 | ||
| Numbers of family members | 4.19 (1.57) | 3.86 (1.90) | 0.142 | ||
| Numbers of children | 2.42 (2.09) | 1.76 (1.60) | 0.003 | ||
| Body Mass Index (Kg/m2) | 29.11 (4.09) | 24.08 (4.59) | 0.000 | ||
| Waist circumference (cm) | 92.13 (9.41) | 78.29 (10.05) | 0.000 | ||
| Glycated hemoglobin A1c (%) | 6.79 (1.51) | 5.84 (0.81) | 0.000 | ||
| Fasting blood sugar (mg/dl) | 133.77 (54.94) | 96.71 (28.87) | 0.000 | ||
| Total cholesterol (mg/dl) | 208.74 (41.57) | 187.74 (37.57) | 0.000 | ||
| Triglyceride (mg/dl) | 202.70 (96.94) | 117.34 (63.54) | 0.000 | ||
| High density lipoprotein (mg/dl) | 50.63 (12.11) | 60.86 (17.93) | 0.000 | ||
| Low density lipoprotein (mg/dl) | 118.24 (36.34) | 104.31 (33.89) | 0.002 | ||
| Systolic blood pressure (mmHg) | 142.50 (18.38) | 122.56 (14.57) | 0.000 | ||
| Diastolic blood pressure (mmHg) | 90.64 (11.51) | 79.26 (10.13) | 0.000 | ||
| Duration of sleep (hrs) | 7.82 (1.44) | 7.95 (1.38) | 0.453 | ||
| Inadequate sleeping status | 7 (8.0) | 19 (10.3) | 0.533 | ||
| Napping | 63 (71.6) | 120 (56.1) | 0.012 | ||
| Meal frequency (3 times/day) | 98 (83.1) | 136 (73.9) | 0.168 | ||
| Skipping breakfast | 5 (5.7) | 15 (7.0) | 0.673 | ||
| Skipping dinner | 3 (3.4) | 20 (9.3) | 0.077 | ||
| Largest meal | 0.484 | ||||
| Breakfast | 18 (20.5) | 29 (15.76) | |||
| Lunch | 48 (54.5) | 120 (56.1) | |||
| Dinner | 9 (10.2) | 32 (15.0) | |||
| Equal meal | 6 (6.8) | 12 (5.6) | |||
| Breakfast & lunch | 0 (0) | 3 (1.4) | |||
| Lunch & dinner | 5 (5.7) | 16 (7.5) | |||
| Breakfast & dinner | 2 (2.3) | 2 (0.9) | |||
| Eating snacks between meals | 49 (55.7) | 112 (52.3) | 0.596 | ||
| Eating home-cooked meal | 88 (100) | 209 (97.7) | 0.148 | ||
| Eating typical Myanmar meal | 85 (96.6) | 206 (96.3) | 0.890 | ||
| Eating meal quickly | 26 (29.5) | 88 (41.1) | 0.059 | ||
| Pay attention to healthy eating | 45 (51.1) | 122 (57.0) | 0.351 | ||
| Eating oily meal | 36 (40.9) | 72 (33.6) | 0.231 | ||
| Eating salty meal | 30 (34.1) | 81 (37.9) | 0.538 | ||
| Eating fruits and vegetables | 86 (97.7) | 205 (95.8) | 0.415 | ||
| Eating fish, meat and eggs | 84 (95.5) | 199 (93.0) | 0.423 | ||
| Drinking caffeinated beverages | 49 (55.7) | 128 (59.8) | 0.508 | ||
| Current smoking | 11 (12.5) | 47 (22.0) | 0.058 | ||
| Past smoking | 20 (22.7) | 51 (23.8) | 0.837 | ||
| Never smoking | 68 (77.3) | 160 (74.8) | 0.645 | ||
| Passive smoker | 33 (37.5) | 99 (46.3) | 0.163 | ||
| Current alcohol drinking | 12 (13.6) | 25 (11.7) | 0.638 | ||
| Past alcohol drinking | 15 (17.0) | 41 (19.2) | 0.668 | ||
| Never alcohol drinking | 71 (80.7) | 172 (80.4) | 0.951 | ||
| Current smokeless tobacco user | 22 (25.0) | 65 (30.4) | 0.349 | ||
| Past smokeless tobacco user | 27 (30.7) | 70 (32.7) | 0.732 | ||
| Never smokeless tobacco user | 61 (69.3) | 140 (65.4) | 0.514 | ||
| Do physical exercises | 32 (36.4) | 75 (35.0) | 0.828 | ||
| Know benefits of physical exercises | 78 (88.6) | 154 (77.1) | 0.022 | ||
| Practice sedentary lifestyle | 70 (79.5) | 169 (79.0) | 0.911 | ||
| Work-related physical activities | 53 (60.2) | 151 (70.6) | 0.081 | ||
| Work-related sedentary lifestyle | 41 (46.6) | 125 (58.4) | 0.055 | ||
| Walking quickly | 29 (33.0) | 96 (44.9) | 0.056 | ||
| Getting weight within 10 years | 66 (75.0) | 113 (52.8) | 0.000 | ||
| Practice health examination | 24 (27.3) | 25 (11.1) | 0.001 | ||
| Family history of NCD | 31 (35.2) | 69 (32.2) | 0.617 | ||
MetS: Metabolic syndrome, NCD: Non-communicable diseases, SD: Standard deviation.
The habit of smoking currently was considerably decreased in MetS group. Regarding laboratory investigations, people with MetS has substantially higher status of all MetS parameters such as FBS, total cholesterol, TG, LDL, and lower HDL. The status of HbA1c, WC and BMI were also increased in people with MetS. The accumulation of MetS components and potential risk factors were compared between male and female (Table 2). There was no remarkable variation between male and female concerning prevalence of MetS, occurrence of MetS components, and accumulation of MetS components. Overall, the half of general population has both one and two components of MetS. While 16% of people owned three components of MetS, 10% had four constituents and other four percent of total population possessed all five risk factors of MetS. Abdominal obesity was the highest component of MetS in female, and it was followed by hyperglycemia, hypertriglyceridemia, and elevated BP. In male, hypertriglyceridemia was the utmost component, and it was followed by hyperglycemia, abdominal obesity, and elevated BP. Low HDL-cholesterol was the lowest component in both sexes.
Table 2.
Prevalence of MetS, components of MetS and potential risk factors.
| Male (%) | Female (%) | P-value | Total (%) | ||
|---|---|---|---|---|---|
| Prevalence of MetS (%) (n = 302) | 27.2 | 30.0 | 0.619 | 29.1 | |
| Accumulation of MetS components (n = 302) | None (%) | 23.9 | 20.0 | 0.503 | 21.2 |
| 1 component (%) | 29.3 | 22.4 | 24.5 | ||
| 2 components (%) | 19.6 | 27.6 | 25.2 | ||
| 3 components (%) | 13.0 | 17.1 | 15.9 | ||
| 4 components (%) | 10.9 | 9.0 | 9.6 | ||
| 5 components (%) | 3.3 | 3.8 | 3.6 | ||
| Prevalence of MetS components (n = 88) | Abdominal obesity | 80.0 | 96.8 | 0.009 | 92.0 |
| Hyperglycemia | 84.0 | 87.3 | 0.684 | 86.4 | |
| Hypertriglyceridemia | 88.0 | 63.5 | 0.023 | 70.5 | |
| Elevated blood pressure | 80.0 | 58.7 | 0.060 | 64.8 | |
| Low HDL cholesterol | 32.0 | 49.2 | 0.143 | 44.3 | |
| Pathophysiological risk factors (n = 302) | |||||
| Diabetes conditions | No diabetes: HbA1c ≤ 6 | 68.5 | 64.3 | 0.474 | 65.6 |
| Prediabetes: HbA1c 6.1–6.5 | 14.1 | 20.0 | 18.2 | ||
| Diabetes: HbA1c > 6.5 | 17.4 | 15.7 | 16.2 | ||
| Hypertensive conditions (Blood pressure, mmHg) | No hypertension: SBP <120 & DBP <80 | 53.3 | 61.4 | 0.047 | 58.9 |
| Prehypertension: SBP 120–139 & DBP 80-89 | 26.1 | 24.3 | 0.739 | 24.8 | |
| Hypertension: SBP ≥140 & DBP ≥90 | 20.7 | 14.3 | 0.167 | 16.2 | |
| Hypercholesterolemia (Total cholesterol level, mg/dl) | Normal <200 | 60.6 | 68.5 | 0.178 | 60.6 |
| Moderately high 200.1–239.9 | 21.2 | 20.7 | 26.2 | ||
| Very high ≥240 | 18.2 | 10.9 | 13.2 | ||
| Obesity conditions, BMI (kg/m2) | Underweight (BMI<18) | 3.3 | 4.3 | 0.001 | 4.0 |
| Normal (BMI 18.01–25.00) | 62.0 | 37.1 | 44.7 | ||
| Overweight (BMI 25.01–30.00) | 23.9 | 35.7 | 32.1 | ||
| Obesity (BMI ≥30.01) | 10.9 | 22.9 | 19.2 | ||
| Abdominal obesity | Normal WC | 71.7 | 44.3 | 0.000 | 52.6 |
| High WC | 28.3 | 55.7 | 47.4 | ||
| Age | 18–40 years | 41.3 | 46.7 | 0.013 | 45.0 |
| 41–60 years | 35.9 | 42.9 | 40.7 | ||
| 61–83 years | 22.8 | 10.0 | 13.9 | ||
| Lifestyle factors | |||||
| Napping | Yes | 64.1 | 59.0 | 0.405 | 60.6 |
| No | 35.9 | 41.0 | 39.4 | ||
| Current smoking | Yes | 54.3 | 3.8 | 0.000 | 19.2 |
| No | 45.7 | 96.2 | 80.8 | ||
| Know benefits of physical exercises | Yes | 75.0 | 82.9 | 0.113 | 80.5 |
| No | 25.0 | 17.1 | 19.5 | ||
| Getting weight within 10 years | Yes | 50.0 | 63.3 | 0.030 | 59.3 |
| No | 50.0 | 36.7 | 40.7 | ||
| Practice health examination | Yes | 14.1 | 17.1 | 0.513 | 16.2 |
| No | 85.9 | 82.9 | 83.8 | ||
BMI: Body mass index, DBP: Diastolic blood pressure, HbA1c: Glycated hemoglobin A1c, HDL: High density lipoprotein, MetS: Metabolic syndrome, SBP: Systolic blood pressure, WC: Waist circumference.
In assessing associated diagnoses, 16.2% of general population has both diabetes and hypertension. Prediabetes was 18.2%, and prehypertension was 24.8%. Overweight and obesity people were 32.1% and 19.2%. Female has significantly higher number of obesity conditions, and it has increased compared with last five years nation-wide data [19]. In contrast, diabetes and hypertensive conditions were not differ between male and female. Lifestyle factors that associate with occurrence of MetS are the practice of midday nap, current smoker, knowing the benefits of physical exercise, getting weight within 10 years and the practice of health examination. Female are notably increased in getting weight within 10 years, and decreased in the number of current smokers. The strength of association between those lifestyle factors and MetS was examined in models developed by logistic regression (Table 3).
Table 3.
Metabolic syndrome odd ratios for potential risk factors, demographic & lifestyle factors (n = 302).
| Model | Included variables | B (SE) | OR | CI | P-value | |
|---|---|---|---|---|---|---|
| Null model | −0.44 (0.12) | 0.65 | 0.000 | |||
| Mode l | Abdominal obesity | Normal WC (Ref) | 1 | |||
| High WC | 3.35 (0.42) | 28.37 | 12.41–64.85 | 0.000 | ||
| Model 2 | Obesity | Normal BMI (Ref) | 1 | |||
| Underweight | −18.88 (11602.71) | 0.00 | 0.99 | |||
| Overweight | 1.97 (0.37) | 7.19 | 3.51–14.74 | 0.000 | ||
| Obesity | 2.82 (0.41) | 16.77 | 7.57–37.16 | 0.000 | ||
| Model 3 | Diabetes conditions | HbA1c ≤ 6 (Ref) | 1 | |||
| HbA1c 6.1–6.5 | 1.09 (0.34) | 2.98 | 1.54–5.75 | 0.001 | ||
| HbA1c > 6.5 | 2.31 (0.36) | 9.95 | 4.93–20.08 | 0.000 | ||
| Model 4 | Hypertensive conditions | No Hypertension (Ref) | 1 | |||
| Pre-hypertension | −0.20 (0.35) | 0.82 | 0.41–1.62 | 0.563 | ||
| Hypertension | 2.19 (0.36) | 8.91 | 4.36–18.20 | 0.000 | ||
| Model 5 | Hypercholesterolemia | No Hypercholesterolemia (Ref) | 1 | |||
| Moderately high | 0.85 (0.31) | 2.34 | 1.31–4.16 | 0.004 | ||
| High | 1.34 (0.37) | 3.82 | 1.87–7.80 | 0.000 | ||
| Model 6 | Hypertriglyceridemia | No hypertriglycedemia (Ref) | 1 | |||
| Hypertriglycedemia | 2.40 (0.29) | 11.05 | 6.20–19.66 | 0.000 | ||
| Model 7 | Age | 18–40 years (Ref) | 1 | |||
| 41–60 years | 1.08 (0.31) | 2.94 | 1.65–5.23 | 0.000 | ||
| 61–83 years | 1.40 (0.39) | 4.06 | 1.91–8.64 | 0.000 | ||
| Model 8 | Napping | No (Ref) | 1 | |||
| Yes | 0.68 | 1.97 | 1.16–3.38 | 0.013 | ||
| Model 9 | Current smoking | No (Ref) | 1 | |||
| Yes | −0.68 (0.36) | 0.51 | 0.25–1.03 | 0.061 | ||
| Model 10 | Know benefits of physical exercises | Yes (Ref) | 1 | |||
| No | −0.84 (0.37) | 0.43 | 0.21–0.91 | 0.024 | ||
| Model 11 | Getting weight within 10 years | No (Ref) | 1 | |||
| Yes | 0.97 | 2.68 | 1.54–4.66 | 0.000 | ||
| Model 12 | Practice health examination | Yes (Ref) | 1 | |||
| No | −1.04 (0.32) | 0.35 | 0.19–0.66 | 0.001 | ||
| Model 13 | Age*Napping | Age (41–60 years)*Napping (Yes) | 1.01 (0.29) | 2.73 | 1.54–4.84 | 0.001 |
| Age (61–83 years)*Napping (Yes) | 1.32 (0.41) | 3.76 | 1.71–8.31 | 0.001 | ||
| Model 14 | Sex*Napping | Sex (Female)*Napping (Yes) | 0.71 (0.26) | 2.04 | 1.23–3.37 | 0.006 |
| Model 15 | Sex*Abdominal obesity | Sex (Female)*Over WC | 3.81 (0.49) | 44.52 | 17.19–115.31 | 0.000 |
| Model 16 | Age*Sex*Napping | Age (41–60 years)*Sex (Female)*Napping (Yes) | 1.01 (0.31) | 2.71 | 1.47–4.98 | 0.001 |
| Age (61–83 years)*Sex (Female)*Napping (Yes) | 2.13 (0.61) | 8.41 | 2.53–27.86 | 0.001 | ||
| Model 17 | Age*Getting weight within 10 years | Age (41–60 years)*Getting weight within 10 years (Yes) | 1.35 (0.29) | 3.84 | 2.17–6.81 | 0.000 |
| Age (61–83 years)* Getting weight within 10 years (Yes) | 2.53 (0.60) | 12.51 | 3.84–40.79 | 0.000 | ||
| Model 18 | Sex*Getting weight within 10 years | Sex (Female)*Getting weight within 10 years (Yes) | 0.61 (0.26) | 1.82 | 1.10–3.01 | 0.019 |
| Model 19 | Getting weight within 10 years*Napping | Getting weight within 10 years (Yes)*Napping (Yes) | 1.06 (0.26) | 2.88 | 1.72–4.80 | 0.000 |
| Model 20 | Sex*Getting weight within 10 years*Napping | Sex (Female)*Getting weight within 10 years (Yes)*Napping (Yes) | 0.98 (0.28) | 2.68 | 1.56–4.59 | 0.000 |
| Model 21 | Age*Getting weight within 10 years*Napping | Age (41–60 years)*Getting weight within 10 years (Yes)*Napping (Yes) | 1.36 (0.34) | 3.91 | 2.02–7.55 | 0.000 |
| Age (61–83 years)* Getting weight within 10 years (Yes)*Napping (Yes) | 2.04 (0.62) | 7.69 | 2.28–25.93 | 0.001 | ||
B: Regression coefficient, CI: Confidence interval, HbA1c: Glycated hemoglobin, OR: Odd ratio, SE: Standard error, WC: Waist circumference.
According to the bivariate analysis, central obesity is the most prominent determinant on the development of MetS. People who have more length in WC (≥80 cm for women and ≥90 cm for men) has the chance of occurring MetS 28 times higher than those with lesser WC. Obesity and overweight people could occur MetS 17 times and seven times higher than their normal counterparts. Having diabetes or hypertension increased the risk of developing MetS nearly 10 times. Hypercholesterolemia is accountable for rising two to three times of MetS while hypertriglyceridemia produces 11 times higher occurrence of MetS. Middle aged and elderly individual have a chance of developing MetS two to four times higher than younger people. In people who are getting weight within last 10 years, they have a risk of facing MetS nearly three times higher. Current smokers, people who knows the benefits of physical exercises, and people who has the practice of checking one's health status are amenable to decreasing the risk of MetS. Therefore, those practices and interaction with other variables were not assessed furthermore. The prevalence of smoking and alcohol drinking are 19% (86% are male) and 12% (97% are male), and smokeless tobacco users are 28% (48% of them are female).
One lifestyle habit that accounts for the rising MetS is the practice of midday nap. People who take a nap during the daytime could occur the condition of MetS two times higher than those without napping practice. Despite the sex is not related directly to MetS, female who have the practice of midday nap increased the risk of MetS two times greater. Middle aged and elderly female who have the practice of midday nap fostered the risk of MetS two to eight times higher. Adults who are getting weight within 10 years had the larger chance of developing MetS four times for middle aged and 13 times for elderly, and it did not depend on sex. If they had the practice of midday nap, the risk of MetS was still remained four to eight times higher. When the sex was included in the interaction with getting weight within 10 years and the practice of midday nap, the risk of MetS reached more to two to three times. Research data for this study can be available at ICPSR data repository [20].
4. Discussion
This study is the first analysis of adult people's lifestyle habits and its association with MetS in Myanmar. With the conduct of community based cross-sectional study, it is discovered that the prevalence of MetS has a similar trend to the conditions of Asia-Pacific region [21]. Comparable to other studies, getting older is remained the same indicator for developing MetS [22] whereas female are not vulnerable to occur MetS in this study. As Myanmar is a lower middle-income country [23], and the highest level of education attainment is completing primary education for study participants, the occupational status and the level of income are also not differed. Therefore, the influence of those predictors on MetS was insignificant though other developed nations in Asia region discovered the association of lower education with MetS [24,25]. Likewise, dietary habits did not produce a remarkable effect on the risk factors of MetS while some studies recognized that the western dietary pattern is associated with higher occurrences of MetS, and fish and vegetables intake produces lower occurrence [26,27]. Nearly all participants in current study consumed Myanmar typical meals, and they also had a same pattern of eating oily and salty meal, consumption of snacks and beverages, and the consistent style of serving frequency, and rapid eating. If the detailed ingredients of Myanmar meal were assessed by a thorough dietary questionnaires as in prior studies, it would provide the different results. The habits of alcohol drinking, smoking and consumption of smokeless tobacco did not yield a profound impact on the association with MetS while there are some controversies in prior findings [9,17]. In the present study, over 80% and 90% of current smoker and current alcohol user are male, and the involvement of male respondents is only 30%. Therefore, those practices on MetS occurrence could not be significant as in earlier studies. Similarly, the practice of physical activity, exercise, and sedentary lifestyle did not show any association with MetS though some public health surveys displayed the good effect of physical workouts. The difference on questionnaire items and the cultural influence could produce such variances [28]. Moreover, family history of NCD was not difference between the two groups. However, people with MetS had the higher percentage of performing health check-up. It could be happended because of their unhealthy conditions.
The daily habit that shows the significant association with MetS is the practice of midday nap. Myanmar people has the custom of taking a nap during daytime, and there are 60% of population in this study who have the practice of midday nap (68% of them are female). The one third of people who take a nap in this study occurred MetS. Napping around the noon is a common practice in some asian countries, and it is considered good for health. However, recent studies identified the association of napping practice with incidence and prevalence of MetS, and type 2 DM [[29], [30], [31]]. In contrast, midday nap is not associated with the prognosis of ischemic heart failure [32]. While there is a consistent U-shaped curve association between short (<6 h) and long (>8 h) nocturnal sleep with ill health, daytime napping with short duration of nighttime sleep has been connected with an increased risk of developing type 2 DM. Despite some explanations for possible mechanism between daytime sleep and glucose metabolism have been explored, a conclusive statement has not yet provided because of scare evidences [33]. In this study, although there is no difference in MetS prevalence between male and female, female who have the practice of midday nap significantly develop MetS greater than male. If those female were aging individuals, they were more vulnerable to acquire MetS than aging individual and female sex separately. In addition, only 11% of people had short nocturnal sleep (<6 h per night), and there was no association between inadequate nighttime sleeper and daytime nappers. Therefore, it could not be said that people who take a nap have inadequate sleep at night. They could have longer sleep pattern because of both adequate night time sleep and extra naps at daytime. However, the data regarding the duration of napping time was not collected in this study. It is difficult to conclude that people with MetS have longer duration of sleep than their counterparts. Further investigations with detailed information on napping practice in wider population are necessary to confirm the relationship between midday napping practice and the risk of MetS.
Among the components of MetS, abdominal obesity or central obesity is the most prominent issue particularly in female and it is congruent with outcomes of previous studies [9,25,34]. Hyperglycemia is the second most common factor and it is also consistent with some outputs of Asia [25]. Despite hypertriglyceridemia is the third most issue for female, it is the first noticeable concern for male in current study, and the same phenomenon has been found in a population-based cohort study [35]. Although a study in a neighbor country found that elevated BP is the most significant risk factor, it is the fourth most frequent issue in this study. The status of HDL cholesterol level is the last common MetS component for male and female, and it is in accordance with previous investigation [8]. According to the findings of present and preceding investigations, it could be said that abdominal obesity of increasing WC is the major risk factor for developing MetS. Some literature already found out the mechanism of visceral obesity and its relatedness to adverse metabolic conditions [8,35]. The decrease in WC is a critical treatment goal for reducing adverse health risks, and it should be routinely measured in health examination program [36]. In this study, WC is significantly related to all MetS parameters, HbA1c and BMI for all participants (Table 4). A simple measurement of WC could produce a valuable indication to the upcoming problem of cardio-metabolic health complications. Some developed nations in Asia used WC as a measurement for their nation-wide health and nutrition survey and it produces much helpful statistics to predict the strength of public health problems. In combination with lifestyle characteristics, the cohort analysis of such annual resources of data could illustrate the cause and effect models [37,38]. In Myanmar, weakness in primary health care and policies on prevention of NCD is still challenging [39]. Additionally, political instability by military coup in February 2021 made extra pressure on health care, and Myanmar people cannot get accessibility to even basic health maintenance [40]. Along with Covid1-19 pandemic issue, many public health problems are waiting for health professionals to solve. This study has provided a foundation for community-based surveillance system to understand the association between socioeconomic factors, health behaviors and cardio-metabolic conditions. Some limitations are concerned in this study. The small number of study participants because of research budget restriction could not provide a more generalizable result. A nature of cross-sectional study could not confirm the causal factors. Using validated and specialized tools for assessing eating habits and physical activity would produce comparable data with other studies than using self-structured general questionnaires. Nevertheless, complete investigation on sociodemographic, lifestyle habits, anthropometrics, and laboratory status of metabolic conditions could generate comprehensive and reliable indicators for cardio-metabolic health conditions of Myanmar people.
Table 4.
Association between WC, BMI, bodyweight, BP and HbA1c, FBS, lipid profiles (n = 302).
| Variables | R-value | P-value |
|---|---|---|
| WC & FBS | 0.25 | 0.000 |
| WC & HbA1c | 0.27 | 0.000 |
| WC & Total cholesterol | 0.28 | 0.000 |
| WC & TG | 0.41 | 0.000 |
| WC & HDL | −0.18 | 0.002 |
| WC & LDL | 0.21 | 0.000 |
| BMI & FBS | 0.16 | 0.006 |
| BMI & HbA1c | 0.19 | 0.001 |
| BMI & Total cholesterol | 0.22 | 0.000 |
| BMI & TG | 0.26 | 0.000 |
| BMI & HDL | −0.11 | 0.059 |
| BMI & LDL | 0.21 | 0.000 |
| Bwt & FBS | 0.13 | 0.020 |
| Bwt & HbA1c | 0.14 | 0.013 |
| Bwt & Total cholesterol | 0.14 | 0.019 |
| Bwt & TG | 0.28 | 0.000 |
| Bwt & HDL | −0.17 | 0.003 |
| Bwt & LDL | 0.10 | 0.100 |
| SBP & FBS | 0.27 | 0.000 |
| SBP & HbA1c | 0.29 | 0.000 |
| SBP & Total cholesterol | 0.28 | 0.000 |
| SBP & TG | 0.30 | 0.000 |
| SBP & HDL | −0.06 | 0.310 |
| SBP & LDL | 0.14 | 0.013 |
| DBP & FBS | 0.20 | 0.001 |
| DBP & HbA1c | 0.18 | 0.002 |
| DBP & Total cholesterol | 0.22 | 0.000 |
| DBP & TG | 0.23 | 0.000 |
| DBP & HDL | −0.04 | 0.487 |
| DBP & LDL | 0.08 | 0.172 |
BMI: Body mass index, Bwt: Bodyweight, DBP: Diastolic blood pressure.
FBS: Fasting blood sugar, HbA1c: Glycated hemoglobin A1c, HDL: High density lipoprotein.
LDL: Low density lipoprotein, TG: Triglyceride, SBP: Systolic blood pressure.
WC: Waist circumference.
5. Conclusions
This study identified the prevalence of MetS and its related factors in small number of Myanmar adult people. In fact, the unhealthy lifestyles and its complication in cardio-metabolic status of wider community residents should be investigated by sustainable and reliable national health examination program. In this way, the impact of NCD in Myanmar could be forecasted and prevented by developing effective public health interventions.
Funding
This work was supported by the Ministry of Health and Sports, Myanmar [grant number 139/2020]. The funding organization did not involve in the conduct of study process including preparation of the research outputs.
CRediT author statement
Su Maw: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing-Original draft, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The author declares that there is no conflicting of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2021.100135.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Alberti K.G.M.M., Eckel R.H., Grundy S.M. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Saklayen M.G. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scholze J., Alegria E., Ferri C. Epidemiological and economic burden of metabolic syndrome and its consequences in patients with hypertension in Germany, Spain and Italy: a prevalence-based model. BMC Publ Health. 2020;10:529. doi: 10.1186/1471-2458-10-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu F., Narimatsu H., Li X. Non-communicable diseases control in China and Japan. Glob Health. 2017;13:91. doi: 10.1186/s12992-017-0315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samson S.L., Garber A.J. Metabolic syndrome. Endocrinol Metab Clin N Am. 2014;43(1):1–23. doi: 10.1016/j.ecl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Kotseva K., EUROASPIRE Investigators The EUROASPIRE surveys: lessons learned in cardiovascular disease prevention. Cardiovasc Diagn Ther. 2017;7(6):633–639. doi: 10.21037/cdt.2017.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyrou I., Tsigos C., Mavrogianni C. Sociodemographic and lifestyle-related risk factors for identifying vulnerable groups for type 2 diabetes: a narrative review with emphasis on data from Europe. BMC Endocr Disord. 2020;20(Suppl 1):134. doi: 10.1186/s12902-019-0463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klongthalay S., Suriyaprom K. Increased uric acid and life style factors associated with metabolic syndrome in Thais. Ethiop J Health Sci. 2020;30(2):199–208. doi: 10.4314/ejhs.v30i2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harikrishnan S., Sarma S., Sanjay G. Prevalence of metabolic syndrome and its risk factors in Kerala, South India: analysis of a community based cross-sectional study. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0192372. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y., Yatsuya H., Iso H., Tamakoshi K., Toyoshima H. Incidence of metabolic syndrome according to combinations of lifestyle factors among middle-aged Japanese male workers. Prev Med. 2010;51(2):118–122. doi: 10.1016/j.ypmed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization Noncommunicable disease country profiles. 2018. https://data.opendevelopmentmekong.net/library_record/noncommunicable-diseases-ncd-country-profiles-2018-myanmar Myanmar. 2018. [DOI] [PubMed]
- 12.Than Yee K., Thwin T., Khin E.E. Metabolic Syndrome in obese and normal weight Myanmar children. J ASEAN Fed Endocr Soc. 2014;28(1):52. [Google Scholar]
- 13.Myanmar Health Research Registry Prevalence of metabolic syndrome among adolescent students in a selected school in North Okkalapa Township, Yangon. http://www.mhrr-mohs.com/index.php?page=researchregistrylist
- 14.World Health Organization Noncommunicable diseases and mental health cluster. surveillance team. 2001. https://www.who.int/ncds/surveillance/steps/Section%204%20Step%202%20Physical%20 Measurements.pdf Section 4: guide to physical measurements (Step 2)
- 15.World Health Organization Noncommunicable diseases and mental health cluster. surveillance team. 2001. https://apps.who.int/iris/handle/10665/68346 STEPS instruments for NCD risk factors (core and expanded version 1.4) : the WHO STEPwise approach to surveillance of noncommunicable diseases (STEPS)
- 16.Help Age International . Ministry of Health and Sports; Naypyitaw: 2017. Training of trainer manual for package of essential non-communicable disease interventions (PEN): together we can prevent and control the world's most common diseases. [Google Scholar]
- 17.Huang P.L. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvard T.H Chan. School of Public Health. Obesity prevention source: abdominal obesity measurement guidelines for different ethnic groups. 2021. https://www.hsph.harvard.edu/obesity-prevention-source/waist-circumference-guidelines-for-different-ethnic-groups/
- 19.Hong S.A., Peltzer K., Lowin K.T., Aung L.S. The prevalence of underweight, overweight and obesity and their related sociodemographic and lifestyle factors among adult women in Myanmar, 2015-16. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0194454. [DOI] [PMC free article] [PubMed] [Google Scholar]; [20] Maw Su Su. Inter-university Consortium for Political and Social Research; Ann Arbor, MI: 2021. Prevalence of metabolic syndrome, its risk factors and associated lifestyles in Myanmar adult people: a community based cross-sectional study. [distributor] 07-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranasinghe P., Mathangasinghe Y., Jayawardena R., Hills A.P., Misra A. Prevalence and trends of metabolic syndrome among adults in the Asia-Pacific region: a systematic review. BMC Publ Health. 2017;17(1):101. doi: 10.1186/s12889-017-4041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klongthalay S., Suriyaprom K. Increased uric acid and life style factors associated with metabolic syndrome in Thais. Ethiop J Health Sci. 2020;30(2):199–208. doi: 10.4314/ejhs.v30i2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The World Bank Poverty report: Myanmar living conditions survey 2017. 2017. https://www.worldbank.org/en/country/myanmar/publication/poverty-report-myanmar-living-conditions-survey-2017
- 24.Ha S., Choi H.R., Lee Y.H. Clustering of four major lifestyle risk factors among Korean adults with metabolic syndrome. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174567. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung G., Jung H., Kim H. Sociodemographic and health characteristics associated with metabolic syndrome in men and women aged ≥50 years. Metab Syndr Relat Disord. 2021;19(3):159–166. doi: 10.1089/met.2020.0051. [DOI] [PubMed] [Google Scholar]
- 26.Agodi A., Maugeri A., Kunzova S. Association of dietary patterns with metabolic syndrome: results from the Kardiovize Brno 2030 study. Nutrients. 2018;10(7):898. doi: 10.3390/nu10070898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan Z., Li Y., Baden M.Y. Association between healthy eating patterns and risk of cardiovascular disease. JAMA Intern Med. 2020;180(8):1090–1100. doi: 10.1001/jamainternmed.2020.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim W.K., Chung W.C., Oh D.J. The effects of physical activity and sedentary time on the prevalence rate of metabolic syndrome and perceived stress in Korean adults. J Exerc Rehabil. 2019;15(1):37–43. doi: 10.12965/jer.1836552.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L., Xu Z., He M. Sleep duration and midday napping with 5-year incidence and reversion of Metabolic Syndrome in middle-aged and older Chinese. Sleep. 2016;39(11):1911–1918. doi: 10.5665/sleep.6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He J., Ouyang F., Qiu D. Association of nap duration after lunch with prevalence of metabolic syndrome in a Chinese government employee population. Int J Environ Res Publ Health. 2020;17(4268) doi: 10.3390/ijerph17124268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Chen L., Shen D. Association of daytime napping in relation to risk of diabetes: evidence from a perspective study in Zhejiang, China. Nutr Metab. 2021;18(18):1–8. doi: 10.1186/s12986-021-00545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salourou M., Archontakis S., Sideris S. The effect of diet, lifestyle and psychological factors in the prognosis of ischemic heart failure. Metabol Open. 2019;1:11–18. doi: 10.1016/j.metop.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cappuccino F.P., Miller M.A. Sleep and cardio-metabolic diseases. Curr Cardiol Rep. 2017;19(110):1–9. doi: 10.1007/s11886-017-0916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luisi C., Figueiredo F.W.D.S., Sousa L.V.A., Quaresma F.R.P., Maciel E.D.S., Adami F. Prevalence of and factors associated with metabolic syndrome in Afro-Descendant communities in a situation of vulnerability in Northern Brazil: a cross-sectional study. Metab Syndr Relat Disord. 2019;17(4):204–209. doi: 10.1089/met.2018.0107. [DOI] [PubMed] [Google Scholar]
- 35.Mansourian M., Babahajiani M., Jafari-Koshki T., Roohafza H., Sadeghi M., Sarrafzadegan N. Metabolic syndrome components and long-term incidence of cardiovascular disease in Eastern Mediterranean Region: a 13-year population-based cohort study. Metab Syndr Relat Disord. 2019;17(7):362–366. doi: 10.1089/met.2018.0136. [DOI] [PubMed] [Google Scholar]
- 36.Ross R., Neeland I.J., Yamashita S. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. 2020;16(3):177–189. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nam G.E., Kim Y., Han K. Obesity fact sheet in Korea, 2018: data focusing on waist circumference and obesity-related comorbidities. J Obes Metab Syndr. 2019;28(4):236–245. doi: 10.7570/jomes.2019.28.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka H., Imai S., Nakade M., Imai E., Takimoto H. The physical examination content of the Japanese national health and nutrition survey: temporal changes. Asia Pac J Clin Nutr. 2016;25(4):898–910. doi: 10.6133/apjcn.092015.34. [DOI] [PubMed] [Google Scholar]
- 39.Latt T.S., Aye T.T., Ko K., Zaw K.K. Gaps and challenges to integrating diabetes care in Myanmar. WHO South East Asia. J Public Health. 2016;5(1):48–52. doi: 10.4103/2224-3151.206553. [DOI] [PubMed] [Google Scholar]
- 40.Aung M.N., Shiu C., Chen W. Amid political and civil unrest in Myanmar, health services are inaccessible. Lancet. 2021;397(10283):1446. doi: 10.1016/S0140-6736(21)00780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.