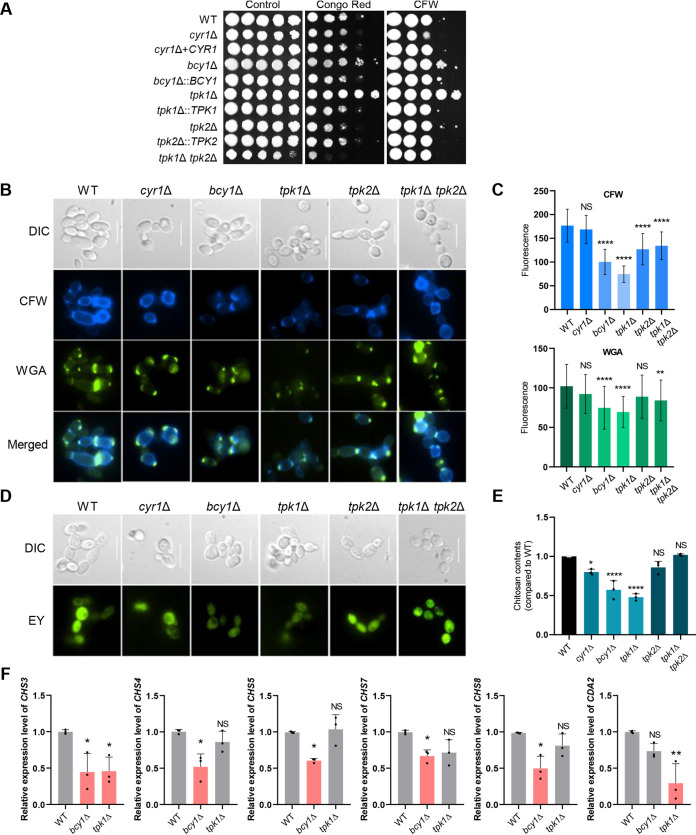
FIG 4.
Role of Cyr1 and PKA in maintaining cell wall integrity of C. auris. (A) WT cells or cells of cyr1Δ (YSBA21), cyr1+CYR1 (YSBA38), bcy1Δ (YSBA4), bcy1Δ::BCY1 (YSBA29), tpk1Δ (YSBA13), tpk1Δ::TPK1 (YSBA36), tpk2Δ (YSBA17), tpk2Δ::TPK2 (YSBA26), tpk1Δ tpk2Δ (YSBA24) C. auris strains were cultured in liquid YPD medium at 30°C overnight, serially diluted (1 to 104), spotted on YPD plates supplemented with 0.01% Congo red (CR) or 0.03 mg/ml calcofluor white (CFW), incubated for 3 days at 30°C, and photographed. One representative image of three independent experiments is shown. (B to E) Assessment of cell wall components of the WT and cAMP/PKA pathway cyr1Δ (YSBA21), bcy1Δ (YSBA4), tpk1Δ (YSBA13), tpk2Δ (YSBA17), and tpk1Δ tpk2Δ (YSBA24) deletion mutant strains. (B and C) Chitin staining. Cells were cultured overnight at 30°C in liquid media, subcultured to OD600 0.8, and stained with FITC-conjugated WGA or CFW. In panel B, a representative image of each strain from fluorescence microscopy with the appropriate filters is shown (scale bar, 10 μm). In panel C, quantitative fluorescence measurements of at least 50 individual cells of each strain, measured using ImageJ/Fiji software, are shown. The data represent means ± the standard deviations (SD). The statistical significance of the difference was determined using one-way ANOVA with Turkey’s multiple-comparison test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; NS, not significant). (D) Chitosan staining. Cells were cultured overnight at 30°C in liquid media, subcultured to OD600 0.8, stained with EY for chitosan, and observed by fluorescence microscopy. (E) Cells were grown at 30°C in liquid media for 2 days, collected by centrifugation, washed, and used in the MBTH assay for the quantitative measurement of chitosan. Three biological replicates are shown. (F) qRT-PCR analysis of C. auris CHS3, CHS4, CHS5, CHS7, CHS8, and CDA2. WT, bcy1Δ (YSBA4), and tpk1Δ (YSBA13) strains were cultured overnight at 30°C, subcultured to OD600 0.8, and extracted for total RNA. The expression level of each gene was normalized with ACT1 as the standard, and the fold change was calculated relative to the basal expression level of fungal cell wall component synthesis-related genes in WT and knockout strains. (The medium was YPD broth containing 1% yeast extract, 2% peptone, and 2% dextrose; the plates contained agar and YPD broth). Three independent biological experiments with three technical replicates were performed. The data represent means ± SEM. The statistical significance of the difference was determined by one-way ANOVA with Bonferroni’s multiple-comparison test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; NS, not significant).