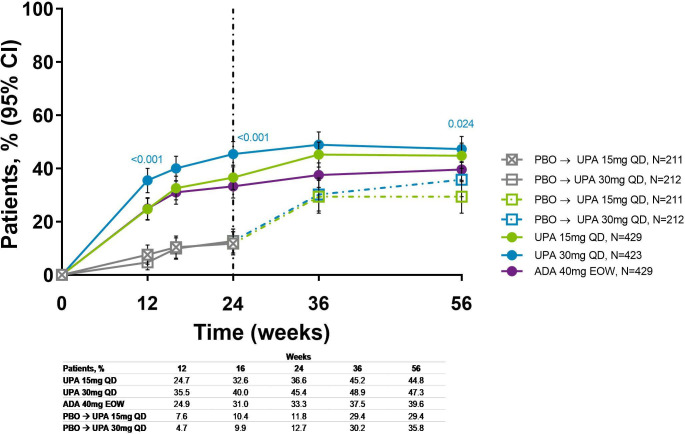
Figure 4.
Proportion of patients achieving MDA over 56 weeks (NRI). Nominal p values are for upadacitinib versus adalimumab. ADA, adalimumab; DMARD, disease-modifying antirheumatic drug; EOW, every other week; MDA, minimal disease activity; NRI, non-responder imputation; PBO, placebo; QD, once daily; UPA, upadacitinib. Patients originally randomised to placebo switched to either upadacitinib 15 mg QD or upadacitinib 30 mg QDy (1:1) at week 24 and their data up to week 24 are under placebo exposure. NRI with additional rescue handling was used, where patients rescued at week 16 are imputed as non-responders. 95% CIs for response rate were calculated based on normal approximation to the binominal distribution. Nominal p value was constructed using Cochran-Mantel-Haenszel test adjusted for the main stratification factor of current DMARD use (yes/no).