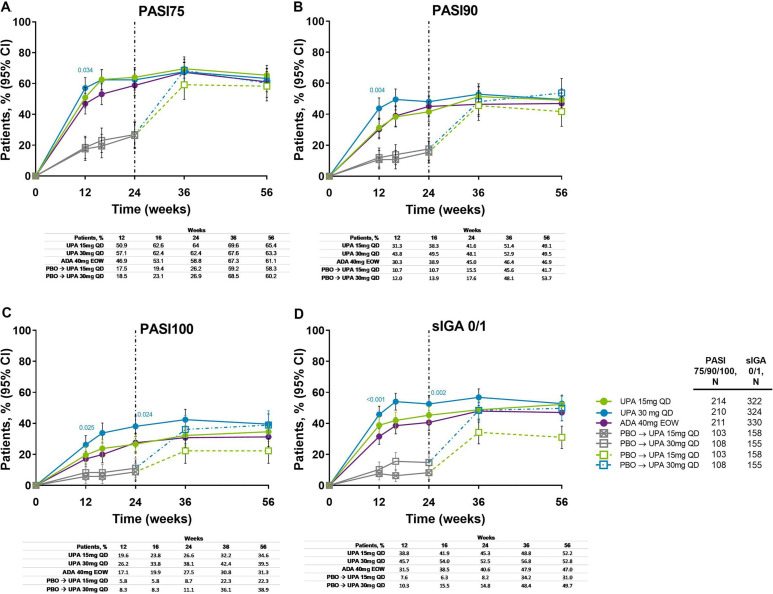
Figure 5.
Proportion of patients achieving (A) PASI75, (B) PASI90, (C) PASI100 and (D) sIGA 0/1 response over 56 weeks (NRI). Nominal p values are for upadacitinib versus adalimumab. ADA, adalimumab; DMARD, disease-modifying antirheumatic drug; EOW, every other week; NRI, non-responder imputation; PASI75/90/100, ≥75%/90%/100% improvement in Psoriasis Area Severity Index; PBO, placebo; QD, once daily; sIGA, Static Investigator Global Assessment of Psoriasis; UPA, upadacitinib. After week 16 assessments have been performed, patients may use concomitant treatments specifically for psoriasis per investigator judgement. Patients originally randomised to placebo switched to either upadacitinib 15 mg QD or upadacitinib 30 mg QD (1:1) at week 24 and their data up to week 24 are under placebo exposure. 95% CIs for response rate were calculated based on normal approximation to the binominal distribution. Nominal p value was constructed using Cochran-Mantel-Haenszel test adjusted for the main stratification factor of current DMARD use (yes/no).