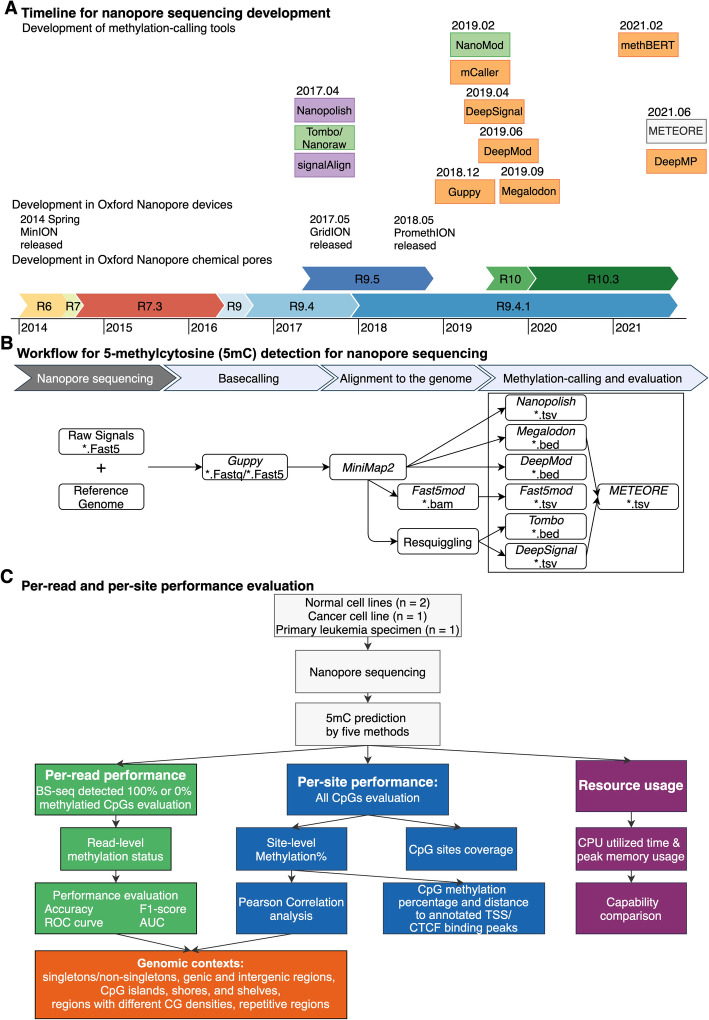
Fig. 1.
Technological development of methylation-calling tools and benchmark strategy. A Timeline of publication and technological developments of Oxford Nanopore Technologies (ONT) methylation-calling tools to detect DNA cytosine modifications. Methylation-calling tools are listed in the order of their publication dates instead of by their bioRxiv online submission dates (except for: BioRxiv date for methBERT and DeepMP, Github repository release time for Megalodon, since these two tools lack an available official publication). Chemical pore versions of Oxford Nanopore flow cells are represented as horizontal-colored bars. Methylation-calling tool are colored by the methylation calling methods (Green: statistical tests, Purple: HMM, Orange: neural network, white: machine learning models). Relevant publication dates are from multiple source [9, 12–23]. B Workflow for 5-methylcytosine (5mC) detection for nanopore sequencing. The analytic pipeline has three steps: (1) Basecalling by Guppy, which requires raw signals and reference genome as input. (2) Alignment to the reference genome by miniMap2 and re-squiggle by Tombo. (3) Methylation calling and evaluation. C Per-read and per-site performance evaluation. We considered the following genomic contexts: singletons, non-singletons, genic and intergenic regions, CpG islands, shores, and shelves, and regions with different CG densities, and repetitive regions. We utilized four nanopore sequencing benchmark datasets and BS-seq datasets as ground truth. We evaluated per-read, per-site performance, the running speed, and computing-memory usage