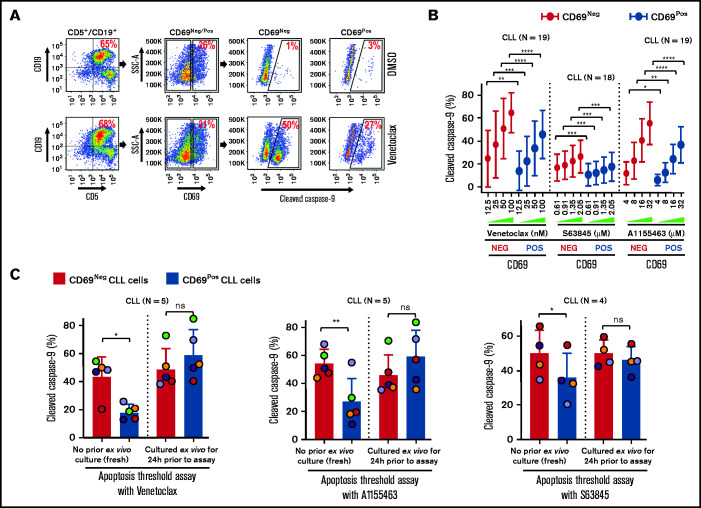
Figure 3.
Circulating CLL cells with CD69+ activation phenotype that overexpress multiple antiapoptotic proteins in vivo display antiapoptotic multidrug resistance. (A-B) Freshly frozen PBMCs from various patients with CLL were screened using the ATA by incubating with inhibitor of Bcl-2 (venetoclax 12.5, 25, 50, or 100 nM), Mcl-1 (S63845 0.61, 0.91, 1.35, or 2.05 µM), or Bcl-xL (A1155463 4, 8, 16, or 32 µM) for 3 hours without added agonists. (A) Representative FCM images of cleaved caspase-9 staining in CD69+ or CD69− CLL (viability dye−/CD5+/CD19+) cells in a patient PBMC (patient 07) incubated with dimethyl sulfoxide (DMSO) or venetoclax. (B) Percentage of CD69+ or CD69− CLL cells positive for cleaved caspase-9 from multiple patient samples exposed to various proapoptotic agents in ATA. (C) To demonstrate ex vivo lability of microenvironmentally induced drug resistance, PBMCs of patients with CLL (patients 8, 25, 27, 41, and 52), either fresh or after culturing ex vivo in RPMI containing 10% fetal calf serum for 24 hours without added agonists, were processed in ATA by incubating with venetoclax (200 nM) or A1155463 (64 µM) for 1 hour (the patient 41 sample was incubated with drugs for 2 hours) or S63845 (6.9 µM) for 3 hours. Since patient 41 was not sensitive to Mcl-1 inhibitor, it was excluded from the S63845 panel. Data are presented as percentage CD69+ or CD69− CLL (viability dye−/CD5+/CD19+) cells positive for cleaved caspase-9. The data in Figure 3B-C are presented after subtracting spontaneous apoptosis values from DMSO treatment controls. Statistical significance was determined by ANOVA with Sidak’s post-hoc test for multiple comparisons. *P < .05; **P < .01; ***P < .001; ****P < .0001. Data are presented as mean ± SD. SSC-A, side scatter area.