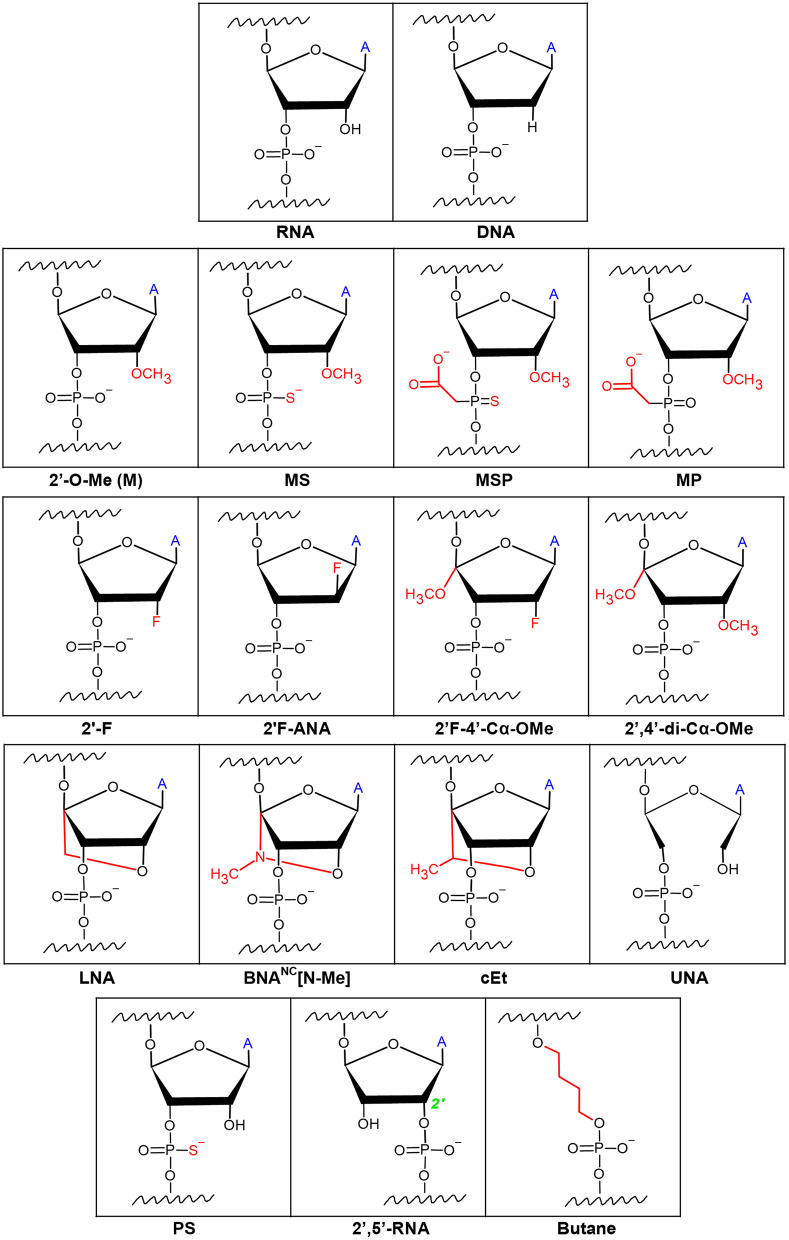
Figure 2.
Chemical modifications on the ribose rings and phosphate backbone of gRNAs. Ribose modifications are typically placed at the 2′OH as it is readily available for manipulation. Simple modifications at the 2′OH include 2′-O-Me, 2′-F, and 2′F-ANA. More extensive ribose modifications such as 2′F-4′-Cα-OMe and 2′,4′-di-Cα-OMe combine modification at both the 2′ and 4′ carbons. Phosphodiester modifications include sulfide-based Phosphorothioate (PS) or acetate-based phosphonoacetate alterations. Combinations of the ribose and phosphodiester modifications have given way to formulations such as 2′-O-methyl 3′phosphorothioate (MS), or 2′-O-methyl-3′-thioPACE (MSP), and 2′-O-methyl-3′-phosphonoacetate (MP) RNAs. Locked and unlocked nucleotides such as locked nucleic acid (LNA), bridged nucleic acids (BNA), S-constrained ethyl (cEt), and unlocked nucleic acid (UNA) are examples of sterically hindered nucleotide modifications. Modifications to make a phosphodiester bond between the 2′ and 5′ carbons (2′,5′-RNA) of adjacent RNAs as well as a butane 4-carbon chain link between adjacent RNAs have been described. ‘A’ symbolizes the nitrogen base of the RNA.