Abstract
Objectives:
Prostate cancer incidence is increasing in the Middle East (ME); however, the data of stage at the diagnosis and treatment outcomes are lacking. In developed countries, the incidence of de novo metastatic prostate cancer ranges between 4% and 14%. We hypothesized that the rates of presentation with advanced disease are significantly higher in the ME based on clinical observation. This study aims to examine the stage at the presentation of patients with prostate cancer at a large tertiary center in the ME.
Methods:
After Institutional Review Board approval, we identified the patients diagnosed with prostate adenocarcinoma and presented to a tertiary care center between January 2010 and July 2015. Clinical, demographic, and pathological characteristics were abstracted. Patients with advanced disease were stratified according to tumor volume based on definitions from practice changing clinical trials. Descriptive and Kaplan–Meier survival analysis was used.
Results:
A total of 559 patients were identified, with a median age at the diagnosis of 65 years and an age range of 39–94 years. Median prostate-specific antigen (PSA) at the presentation was 10 ng/ml, and almost a quarter of the men (23%) presented with metastatic disease. The most common site of metastasis was the bone (34/89, 38%). High-volume metastasis was present in 30.3%, 9%, and 5.2% of the cohort based on STAMPEDE, CHAARTED, and LATITUDE trial criteria, respectively.
Conclusion:
This is the first report showing the high proportion of men from ME presenting with de novo metastasis. This could be due to many factors, including the highly variable access to specialist multidisciplinary management, lack of awareness, and lack of PSA screening in the region. There is a clear need to raise the awareness about prostate cancer screening and early detection and to address the rising burden of advanced prostate cancer affecting men in the ME region.
Keywords: Cancer staging, Middle East, prostate cancer, prostate neoplasm, tumor staging
INTRODUCTION
During the past decades, there have been large changes in the incidence, presentation, and management of prostate cancer. In developed countries, the incidence of prostate cancer has been declining,[1,2] with de novo metastatic prostate cancer ranging between 4% and 14%.[3,4] In the Middle East (ME), the incidence of prostate cancer has been consistently increasing over the last decade.[1,5,6]
Recommendations against prostate-specific antigen (PSA) screening by the US Preventive Services Task Force (USPSTF) in 2008 for men above 75 and in 2012 for all men led to reduction in prostate cancer incidence in the US. However, the US Surveillance Epidemiology and End Results (SEER) Collaborative Stage data demonstrated a stage migration as the patients presenting with distant metastasis has increased in the period between 2008 and 2013.[7] In 2018, the USPSTF revisited their recommendations, suggesting that men age 55–69 years should make an individualized decision regarding PSA screening with their clinician, screening is still not recommended for men over 70.[8] In the ME, PSA screening is not widely adopted, and in the absence of well-established regional registries, there are no data to reflect the disease stage in the region.
Interest in studying the epidemiology of prostate cancer stage at the diagnosis in different populations has gained new momentum with a paradigm shift in the way that patients presenting with advanced disease are treated. Notably, the CHAARTED, STAMPEDE, and LATTITUDE trials have redefined the management for de novo metastatic prostate cancer as these trials demonstrated improvement in the survival with the addition of systemic therapy with either docetaxel or abiraterone to androgen deprivation therapy (ADT).[9,10,11,12] Recently, enzalutamide and apalutamide were found to improve the survival in hormone-sensitive metastatic disease.[13,14] In an attempt to mitigate the disease burden in the ME region and help stakeholders to implement new policies to improve patient outcomes, it is crucial to study the disease stage at initial presentation. We sought to assess the stage at the presentation of patients with prostate cancer at a large tertiary center in the ME.
METHODS
After institutional review board approval, we identified all the cases of prostate cancer diagnosed and treated at a tertiary care center between January 2010 and July 2015. The stage at the diagnosis was recorded according to the American Joint Commission on Cancer staging manual 8th addition.[15] Clinical, demographic, and pathological characteristics from the patient charts were abstracted. Patients with advanced disease were stratified according to tumor volume as defined in recent large trials, namely STAMPEDE, CHAARTED, and LATITUDE [Table 1].[9,10,11,12]
Table 1.
Three of the randomized control trials for patients with advanced prostate cancer
| Study | Criteria for high-volume disease | Reference | Patients from our study who fit the criteria, n (%) |
|---|---|---|---|
| CHAARTED | Presence of visceral metastasis and/or at least 4 bone metastases with at least 1 beyond the pelvis or vertebral column | [10] | 47 (9) |
| STAMPEDE | Either metastatic or node-positive or high-risk locally advanced disease with at least 2 of stage T3/T4, PSA >40 ng/ml or Gleason score 8–10 | [10,11] | 158 (30.3) |
| LATTITUDE | Newly diagnosed hormone-sensitive metastatic prostate cancer with at least two of: Gleason score ≥8, ≥3 bone lesions or measurable visceral lesions | [12] | 27 (5.2) |
PSA: Prostate-specific antigen
We analyzed the data using the SPSS version 24, IBM Corp, Armonk, NY, USA. First, descriptive analysis was conducted to describe the distribution of demographic data, presentations, and biochemical laboratory values. We then conducted Kaplan–Meier survival analysis to calculate the median overall survival.
RESULTS
A total of 559 patients were identified, with a median age of 65 years and ranging between 39 and 94 years. Median BMI of all patients was 28.2 kg/m2 (range 22.3–40.3 kg/m2). Overall, 86 (15.4%) patients had a positive family history of prostate cancer. The mean initial PSA for all patients was 77.4 ng/ml and median PSA was 10 ng/ml. Of these, 522 (93.4%) of patients had staging data at the time of presentation. The number and proportion of these patients presenting with Stages 1, 2, 3, 4A, and 4B were 65 (11.6%), 233 (41.7%), 105 (18.8%), 30 (5.4%), and 89 (15.9%), respectively. Regarding Gleason Groups, 137 (24.5%) patients presented with a Gleason Group 1, 129 (23.1%) presented with Gleason Group 2, 94 (16.8%) with Gleason Group 3, 96 (17.2%) with Gleason Group 4, and 66 (11.8%) with Gleason Group 5 [Table 2]. As part of the standard treatment of locally advanced prostate cancer, 240 patients underwent radiation therapy and 208 patients underwent radical prostatectomy. Fifty-five patients (26.4%) had a robotic prostatectomy and 153 (73.56%) had an open prostatectomy. There was no difference between the median ages of each group.
Table 2.
Epidemiology of the patient population
| Demographics | n (%) |
|---|---|
| Nationality, n (%) | |
| Lebanese | 399 (71.4) |
| Syrian | 32 (5.7) |
| Iraqi | 64 (11.4) |
| Other | 27 (4.8) |
| Missing | 37 (6.6) |
| Total | 559 |
| Median age (range) | 65 (39–94) |
| Stage at presentation, n (%) | |
| 1 | 65 (11.6) |
| 2 | 233 (41.7) |
| 3 | 105 (18.8) |
| 4A | 30 (5.4) |
| 4B | 89 (15.9) |
| Missing | 37 (6.6) |
| Gleason group, n (%) | |
| 1 | 137 (24.5) |
| 2 | 129 (23.1) |
| 3 | 94 (16.8) |
| 4 | 96 (17.2) |
| 5 | 66 (11.8) |
| Missing | 37 (6.6) |
| Median BMI (kg/m2) (range) | 28.2 (22.3–40.3) |
| Family history of prostate cancer, n (%) | |
| Yes | 86 (15.4) |
| No | 340 (60.8) |
| Missing | 133 (23.8) |
BMI: Body mass index
Metastatic disease
Bone metastasis alone at the presentation was observed in 38.2% of patients. 28.1% of patients had lymph node and bone involvement and 13.5% of patients had lymph nodes, visceral, and bone metastasis at presentation[Table 3], making bone the most common site of metastasis with 77% of Stage 4 patients presenting with bone metastatic prostate cancer. Among the patients who presented with distant metastases, 60 patients (67.4%) had high-volume disease, whereas 24 (27.0%) had low-volume disease [Table 4].
Table 3.
Location of metastasis at presentation for Stage 4 patients
| Metastasis type | Number of patients, n (%) |
|---|---|
| Distant LN only | 7 (7.87) |
| Visceral only | 3 (3.37) |
| Bone only | 34 (38.20) |
| Distant LN + visceral | 2 (2.25) |
| Distant LN + bone | 25 (28.09) |
| Bone + visceral | 4 (4.49) |
| Distant LN + visceral + bone | 12 (13.48) |
| Missing | 2 (2.25) |
| Total | 89 (100) |
LN: Lymph node
Table 4.
High versus low volume of metastasis at presentation
| Disease burden | Number of patients, n (%) |
|---|---|
| High volume | 60 (67.4) |
| Low volume | 24 (27.0) |
| Missing | 5 (5.6) |
In our study population, 158 patients (30.3%), 47 (9%), and 27 (5.2%) met the inclusion criteria for the STAMPEDE, CHAARTED, and LATITUDE trials, respectively [Table 1].
Regarding the subcohort that met STAMPEDE trial inclusion criteria, the median follow-up of our patients who fit the high-risk criteria was 20 months with median survival of 119 months, in comparison to trial patients who had median follow-up of 40 months and 3-year survival of 83%. For the subcohort that met CHAARTED study inclusion criteria, our patients had median follow-up of 20 months and median survival of 119 months, compared to the median follow-up of 28.9 months and median survival of 57.6 months in the trial patients. Finally, our patients who met the high-risk criteria as defined by the LATITUDE trial had a median follow-up of 15 months and median survival of 38 months, in comparison to the trial patients, who had median follow-up of 51.8 months and median overall survival of 53.3 months [Table 5.
Table 5.
Mean survival for patients meeting STAMPEDE, CHARTED, and LATITUDE trial criteria
| Trial | Number of patients | Median follow-up time of our study (months) | Median follow-up time of trial (months) | Median survival time of our study (months) | Median survival time of trial (months) |
|---|---|---|---|---|---|
| STAMPEDE | 158 | 20 | 40 | 119 | (only 3-year survival available) |
| CHAARTED | 47 | 20 | 28.9 | 119 | 57.6 |
| LATITUDE | 27 | 15 | 51.8 | 38 | 53.3 |
We divided patients into two groups based on their age. From the 559 patients reviewed, 430 patients (76.9%) were younger than 75 years and 129 (23.1%) were older than 75 years. In both the younger and older groups, the highest proportion of patients presented with Stage 2 disease (43.3% and 36.4%, respectively). The proportion of patients presenting with Stage 4 disease in the younger group was 17.0% compared to 35.7% in the older group.
Nationality and stage of presentation
The majority of the patients (339, 71.4%) were Lebanese, 32 (5.7%) were Syrian, 64 (11.4%) were Iraqi, and 27 (4.8%) were of other nationalities. Lebanese and Syrian patients presented mainly with Stage 2 disease accounting for 44.1% and 37.5%, respectively, in the two populations, whereas 48.5% of Iraqi patients presented with Stage 4 disease, with 42.2% presenting with Stage 4B [Figure 1].
Figure 1.
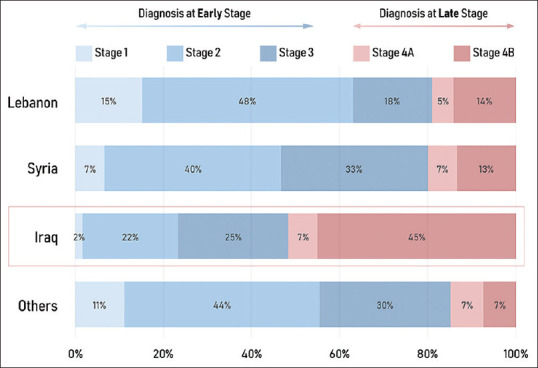
Epidemiology of the patient population stratified by stage
DISCUSSION
Our study identified 559 patients presenting with prostate cancer to a tertiary care center between 2010 and 2015. Of the 522 patients with staging data available, a significant proportion of these presented with Stage 4 disease (22.7%), including 17% with distant metastasis at presentation. Conversely, the US National Cancer Database collected between 2004 and 2013 and the SEER database between 2007 and 2012 found that only 3% and 6.4% of these patients had metastasis at the diagnosis, respectively.[16] While these values are much lower than those found in our study, a study from the UK found comparable results, with 17%–34% of prostate cancer patients had metastasis at the diagnosis.[14,17] Of note, our study found that 48.5% of Iraqi patients presented with Stage 4 disease. The higher proportion of late stage patients at our center can be explained by multiple factors, including but not limited to: wide scale screening for prostate cancer has never been adopted in the ME given the lack of medical infrastructure supporting primary care interventions including cancer screening. The medical center where this study has been conducted is a large tertiary care center receiving referrals for the most challenging cases in the region, especially expatriated patients who travel to seek excellence in medical care.[18,19] Conversely, our center is a large referral for prostate cancer surgery and specialist radiation therapy, which may have skewed the demographics of the cohort toward patients with localized disease.
In our study, the mean initial PSA for all patients was 77.4 ng/ml, median PSA was 10 ng/ml. This is in contrast to a study of 230,081 patients in the US that found the mean PSA to be 26.4 ng/ml and median to be 5.3 ng/ml.[20] This higher level of PSA observed in our patients is in accordance with a study from Kuwait which found that Middle Eastern men have higher PSA levels compared to the US and Europe.[21] These data and another study showed that PSA levels above 10 ng/mL in Arab men were more likely to be due to benign prostatic hyperplasia with prostatitis compared to similar levels in American or European men.[22]
We stratified our cohort according to age, similar to a recent US study which showed that 83.1% of the younger population presented with a Gleason of 7 or less compared to 64.1% of the older population.[7] Our study found that 75.4% of the younger population compared to 53.2% of the older population presented with a Gleason of 7 or less. In both age groups, a smaller proportion of our patients, compared to the US study, had a lower Gleason score, again emphasizing that our patients tend to present with higher stage disease. In our study, 19.8% of the younger population and 26.1% of the older population presented with a Gleason score of 8 or higher.
Patients presenting with Stage 4 disease were significantly older than those presenting at earlier stage, with a mean age of 71.4 years. In the US, the average age of patients with Stage 4 disease has been decreasing over the years, with the average age being 71.9 between 1988 and 1992, 70.9 between 1993 and 1997, and 68.7 between 1998 and 2003.[23]
For those patients de novo metastatic disease at presentation, the most common location of the metastasis was the bone only (38.2%), followed by lymph node (s) and bone (28.09%). Only 3.37% of patients presented with visceral metastasis alone. This is in accordance with many studies, which show bone to be the most common location of metastasis, followed by lymph nodes.[24]
We collected the data from patients treated prior to the publication of three landmark trials that have reshaped the treatment sphere for advanced prostate cancer. According to these trials, patients with advanced disease if identified early for high-risk features might benefit from a more aggressive treatment approach. The number of high-risk patients who met criteria for STAMPEDE, CHAARTED, and LATITUDE trials were 158 (30.3%), 47 (9%), and 27 (5.2%) [Table 3]. The prolonged survival times seen in our “high-risk” patients who did not receive new practice-changing therapies is likely due to shorter median follow-up times of our study. The control arms of the three trials had median overall survival times of 40, 47.2, and 34.7 months, respectively.
With the rapidly advancing treatment algorithms for prostate cancer and increasing treatment costs, it is crucial to have population-specific data to enable health-care systems planning. Our group have recently reported the first regional consensus on resource-stratified prostate cancer management in the ME.[25]
The limitations of our study include the fact that our sample consists of patients presenting to a single tertiary care center, limiting the generalizability to the Middle Eastern region as a whole. Furthermore, our research was limited by the staging data available, and a large number of our patients were lost to follow-up or with limited data on treatment outcome. We have identified an urgent need for improved local and regional cancer registry data collection including stage at diagnosis and treatment outcomes.
This is the first report that highlights the high proportion of patients with prostate cancer who present with late stage disease to a tertiary care center in Lebanon. This high percentage could be due to many factors including the highly variable access to specialist multidisciplinary management, lack of awareness and lack of PSA screening in the region. With new trials showing improved outcomes for patients with high-risk disease with the addition of systemic therapies to ADT, it is important for these practice-changing therapies to be applied to our own population, which sees a large number of these high-risk patients. Furthermore, there is a clear need to raise the awareness about prostate cancer screening and early detection. Our data highlight the need to address the rising burden of advanced prostate cancer affecting men in the ME region.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to acknowledge that some data from this study has previously been published in an abstract in the Journal of Clinical Oncology: Prostate cancer stage at diagnosis: First data from a Middle-Eastern cohort. DOI: 10.1200/JCO.2017.35.6_suppl.e552 Journal of Clinical Oncology 35, no. 6_suppl (February 20, 2017) e552-e552.
REFERENCES
- 1.Fleshner K, Carlsson SV, Roobol MJ. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol. 2017;14:26–37. doi: 10.1038/nrurol.2016.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Westerberg M, Franck Lissbrant I, Damber JE, Robinson D, Garmo H, Stattin P. Temporal changes in survival in men with de novo metastatic prostate cancer: Nationwide population-based study. Acta Oncol. 2020;59:106–11. doi: 10.1080/0284186X.2019.1662084. [DOI] [PubMed] [Google Scholar]
- 4.Helgstrand JT, Røder MA, Klemann N, Toft BG, Lichtensztajn DY, Brooks JD, et al. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer-A population-based analysis of 2 national cohorts. Cancer. 2018;124:2931–8. doi: 10.1002/cncr.31384. [DOI] [PubMed] [Google Scholar]
- 5.Hilal L, Shahait M, Mukherji D, Charafeddine M, Farhat Z, Temraz S, et al. Prostate cancer in the Arab world: A view from the inside. Clin Genitourin Cancer. 2015;13:505–11. doi: 10.1016/j.clgc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Shamseddine A, Saleh A, Charafeddine M, Seoud M, Mukherji D, Temraz S, et al. Cancer trends in Lebanon: A review of incidence rates for the period of 2003-2008 and projections until 2018. Popul Health Metr. 2014;12:4. doi: 10.1186/1478-7954-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu JC, Nguyen P, Mao J, Halpern J, Shoag J, Wright JD, et al. Increase in prostate cancer distant metastases at diagnosis in the United States. JAMA Oncol. 2017;3:705–7. doi: 10.1001/jamaoncol.2016.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, Davidson KW, Doubeni CA, Ebell M, Epling JW, Jr, Kemper AR, Krist AH, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Siu AL, Tseng CW US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement? JAMA. 2018;319:1901–13. doi: 10.1001/jama.2018.3710. doi: 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James ND, de Bono JS, Spears MR, Clarke NW, Mason, Dearnaley DP, et al. STAMPEDE Investigators. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377:338–51. doi: 10.1056/NEJMoa1702900. doi: 10.1056/NEJMoa1702900. Epub 2017 Jun 3. PMID: 28578639; PMCID: PMC5533216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–60. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 13.Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–31. doi: 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 14.Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 15.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–9. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 16.Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004-2013) Prostate Cancer Prostatic Dis. 2016;19:395–7. doi: 10.1038/pcan.2016.30. [DOI] [PubMed] [Google Scholar]
- 17. [[Last accessed on Jan 2021]]. Available from: https://www.cancerresearchuk.org/healthprofessional/cancer-statistics/statistics-by-cancer-type/prostatecancer/incidence .
- 18.Arafa MA, Rabah DM, Wahdan IH. Awareness of general public towards cancer prostate and screening practice in Arabic communities: A comparative multi-center study. Asian Pac J Cancer Prev. 2012;13:4321–6. doi: 10.7314/apjcp.2012.13.9.4321. [DOI] [PubMed] [Google Scholar]
- 19.Shahait M. Prostate cancer management in the Middle East. World J Urol. 2020;38:2063–4. doi: 10.1007/s00345-019-02892-7. [DOI] [PubMed] [Google Scholar]
- 20.MacKintosh FR, Sprenkle PC, Walter LC, Rawson L, Karnes RJ, Morrell CH, et al. Age and prostate-specific antigen level prior to diagnosis predict risk of death from prostate cancer. Front Oncol. 2016;6:157. doi: 10.3389/fonc.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kehinde EO, Sheikh M, Mojimoniyi OA, Francis I, Anim JT, Nkansa-Dwamena D, et al. High serum prostate-specific antigen levels in the absence of prostate cancer in Middle-Eastern men: The clinician's dilemma. BJU Int. 2003;91:618–22. doi: 10.1046/j.1464-410x.2003.04199.x. [DOI] [PubMed] [Google Scholar]
- 22.Sheikh M, Al-Saeed O, Kehinde EO, Sinan T, Anim JT, Ali Y. Utility of volume adjusted prostate specific antigen density in the diagnosis of prostate cancer in Arab men. Int Urol Nephrol. 2005;37:721–6. doi: 10.1007/s11255-005-4683-2. [DOI] [PubMed] [Google Scholar]
- 23.Cetin K, Beebe-Dimmer JL, Fryzek JP, Markus R, Carducci MA. Recent time trends in the epidemiology of stage IV prostate cancer in the United States: Analysis of data from the surveillance, epidemiology, and end results program. Urology. 2010;75:1396–404. doi: 10.1016/j.urology.2009.07.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandaglia G, Abdollah F, Schiffmann J, Trudeau V, Shariat SF, Kim SP, et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate. 2014;74:210–6. doi: 10.1002/pros.22742. [DOI] [PubMed] [Google Scholar]
- 25.Mukherji D, Youssef B, Dagher C, El-Hajj A, Nasr R, Geara F, et al. Management of patients with high-risk and advanced prostate cancer in the Middle East: Resource-stratified consensus recommendations. World J Urol. 2020;38:681–93. doi: 10.1007/s00345-019-02872-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


