Abstract
Introduction:
The association between inflammation and malignancies is being recognized. In this study, we assessed the use of preoperative neutrophil–lymphocyte ratio (NLR) and lymphocyte–monocyte ratio (LMR) in predicting cancer-specific survival (CSS) and inguinal node involvement in patients with carcinoma penis.
Methods:
Sixty-nine patients operated for squamous cell carcinoma penis with inguinal node dissection between 2012 and 2020 were identified. We recorded the type of surgery (partial/total penectomy), T stage, grade, lymphovascular invasion (LVI), perineural invasion (PNI), pathological status of inguinal nodes and nodal stage (pN1–3), extranodal extension (ENE), and CSS. The hemogram performed within 2 weeks of surgery was used for calculating NLR and LMR.
Results:
Partial penectomy was the most common surgery (65.22%) and pT2 was the most common stage (53.62%). Grade 2 was seen in 66.67%, LVI in 34.78%, PNI in 37.68%, 52.17% had inguinal node involvement with pN3 being the most common (36.23%), and 36.23% had ENE. Kaplan–Meier analysis revealed that NLR of >3 and the LMR ≤3 indicated an inferior CSS (P = 0.05 and 0.04, respectively). T stage, inguinal node involvement, LVI, pN stage, and ENE were also associated with inferior CSS (P < 0.05). On multivariate analysis, T stage was significantly associated with CSS (P = 0.02). The NLR >3 and LMR ≤3 were also significantly associated with the presence of pathological inguinal node involvement (P = 0.001 and 0.026).
Conclusion:
NLR and LMR may help in predicting CSS and inguinal node involvement in patients of carcinoma penis.
Keywords: Cancer-specific survival, inflammation, lymph nodes, metastasis, penile cancer
INTRODUCTION
Penile cancer is an uncommon malignancy with an overall incidence of 0.84 cases per 100,000.[1] It is an aggressive malignancy, especially when there is a lymph nodal spread. Other factors that have been shown to affect the outcomes are T stage, the grade of tumor differentiation, lymphovascular invasion (LVI), N stage, etc.[2] A few others, such as p53 expression, programmed death ligand 1 overexpression, and Ki-67, have also been shown to have prognostic implications, though their clinical efficacy has been shown to be limited.[3]
There is increasing evidence that supports the association of the development of solid organ malignancy with the presence of systemic inflammation. The makers of inflammation are being identified that can help in prognostication of the patients with cancer.[4] A few biomarkers have been found to be associated with outcome such as C-reactive protein, neutrophil–lymphocyte ratio (NLR), and platelet–lymphocyte ratio.[3] Of these, the ratios are easiest to perform on a routine preoperative blood sample and they have been shown to have prognostic implication in a variety of cancers. In patients of carcinoma penis, due to the rarity of the disease, very limited studies are available in the literature that evaluate the prognostic value of these ratios.[3,5,6,7,8,9] In the present study, we aim to assess the value of preoperative NLRs in predicting the cancer-specific survival (CSS) and pathological involvement of inguinal nodes in patients of squamous cell carcinoma of penis who had undergone bilateral inguinal lymph node dissection. We also assess the predictive value of a new parameter, the lymphocyte–monocyte ratio (LMR) which has previously been assessed in only one study of patients with carcinoma penis.[9] In addition, we also analyze the effects of various histopathological factors and pathological stage on the outcome.
METHODS
Patient selection
Approval from the institutional review board was obtained for this study. A retrospective analysis of a prospectively recorded database was performed to identify the patients who had been operated for carcinoma penis from 2012 to 2020. In this population, the patients who had undergone a bilateral inguinal lymph node dissection, indicated due to the clinical staging, were identified. We recorded the information including the type of surgery for the penile lesion (partial or total penectomy), stage of the penile tumor (pT1–4), pathological grade (Grade 1–3), presence or absence of LVI, perineural invasion (PNI), the pathological status of the inguinal nodes (positive or negative), pathological nodal stage (pN1–3), presence of extranodal extension (ENE), receipt of adjuvant chemotherapy, and CSS. The pathological classification was done on the basis of the Eighth Edition of the American Joint Committee on Cancer TNM staging system. The reports of the hemograms of all these patients were retrieved from the database. The hemogram that was performed within 2 weeks of surgery was taken into consideration for calculating the NLR and LMR. If there were multiple reports, the one which was done closest to the date of surgery was selected for the analysis. All the patients who did not have the complete aforementioned information or had a histology other than squamous cell carcinoma or had distant metastasis at the time of surgery or any history of prior radiotherapy or neoadjuvant chemotherapy were excluded from the analysis. The recurrences were recorded as locoregional or distant. The period of CSS was calculated from the point of surgery to the death due to malignancy.
Statistical analysis
The statistical analysis was done using the Statistical Package for the Social Sciences (SPSS) version 25 (International Business Machines Corporation, New York, U.S.A). The median and ranges were calculated for continuous variables, whereas proportions and frequency tables were used to summarize categorical variables. The level of significance was considered as P < 0.05. Kaplan–Meier curves were generated along with log-rank analysis in order to estimate the CSS for each of the variables. The cutoff value for the NLR and LMR cutoff in our analysis was taken to be 3 which was in agreement with previously reported studies.[5,6,9] Multivariate analysis was done using linear regression test.
RESULTS
Description of the cohort
A total of 126 patients were treated for carcinoma penis during the study period, of which 78 patients had a bilateral inguinal lymph node dissection. We included a total of 69 patients in our study who fulfilled the inclusion and the exclusion criteria. On analysis, it was seen that partial penectomy was the most common surgery performed (65.22%) and pT2 was the most common stage which was seen in 53.62% of the patients. The pathological Grade 2 was seen most often (66.67%), LVI was seen in 34.78%, and PNI in 37.68%. 52.17% of the patients had inguinal lymph node involvement. The pelvic lymph node dissection was carried out in 22 patients. The distribution of pN1, pN2, and pN3 was 11.59%, 4.35%, and 36.23%, respectively. ENE was seen in 36.23% of the patients [Table 1]. Adjuvant chemotherapy was administered to five patients. Locoregional recurrences were seen in nine patients during the follow-up period while five had distant metastasis. The median CSS of patients alive at the time of analysis was 18 months (range: 2–74 months).
Table 1.
Clinicopathological characteristics of the patients with results of univariate and multivariate analysis of the predictive value of hematologic and pathological variables for cancer specific survival and involvement of groin nodes
| Variable | Frequency (%) | N/L>3 (%) | L/M>3 (%) | Cancer specific survival (P) | Groin node involvement (P) | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Univariate | Multivariate | Univariate | Multivariate | ||||
| Neutrophil/lymphocyte ratio | |||||||
| ≦3 | 29 (42.03) | - | - | 0.05 | 0.94 | 0.001 | 0.09 |
| >3 | 40 (57.97) | - | - | ||||
| Lymphocyte/monocyte ratio | |||||||
| ≦3 | 45 (59.42) | - | - | 0.04 | 0.95 | 0.02 | 0.32 |
| >3 | 24 (40.58) | - | - | ||||
| Type of surgery | |||||||
| Partial penectomy | 45 (65.22) | 21 (46.67) | 18 (40) | 0.76 | - | 0.31 | - |
| Total penectomy | 24 (34.78) | 19 (79.17) | 6 (25) | ||||
| pT stage | |||||||
| pT1 | 15 (21.74) | 6 (40) | 8 (53.33) | 0.002 | 0.02 | 0.75 | - |
| pT2 | 37 (53.62) | 20 (54.05) | 13 (35.13) | ||||
| pT3 | 16 (23.19) | 13 (81.25) | 3 (18.75) | ||||
| pT4 | 1 (1.45) | 1 (100) | |||||
| Grade of tumor | |||||||
| G1 | 4 (5.80) | 1 (25) | 3 (75) | 0.25 | - | 0.07 | - |
| G2 | 46 (66.67) | 24 (52.17) | 16 (34.78) | ||||
| G3 | 19 (27.54) | 15 (78.95) | 15 (78.95) | ||||
| Lymphovascular invasion | |||||||
| Yes | 24 (34.78) | 18 (75) | 8 (33.33) | 0.03 | 0.68 | <0.001 | 0.007 |
| No | 45 (65.22) | 22 (48.89) | 16 (35.56) | ||||
| Perineural invasion | |||||||
| Yes | 26 (37.68) | 18 (69.23) | 9 (34.62) | 0.052 | - | 0.02 | 0.99 |
| No | 43 (62.32) | 22 (51.16) | 15 (34.88) | ||||
| Pathologic nodal status | |||||||
| Positive | 36 (52.17) | 28 (77.78) | 8 (22.22) | <0.001 | 0.95 | - | - |
| Negative | 33 (47.83) | 12 (36.36) | 16 (48.48) | ||||
| pN stage | |||||||
| pN0 | 33 (47.83) | 12 (36.36) | 16 (48.48) | <0.001 | - | - | - |
| pN1 | 8 (11.59) | 8 (100) | 3 (37.5) | ||||
| pN2 | 3 (4.35) | 2 (66.67) | 1 (33.33) | ||||
| pN3 | 25 (36.23) | 18 (72) | 4 (16) | ||||
| Extranodal extension | |||||||
| Yes | 25 (36.23) | 18 (72) | 4 (16) | <0.001 | 0.18 | - | - |
| No | 44 (63.77) | 22 (50) | 20 (45.45) | ||||
N/L: Neutrophil/lymphocyte, L/R: Lymphocyte/monocyte
Analysis of cancer-specific survival
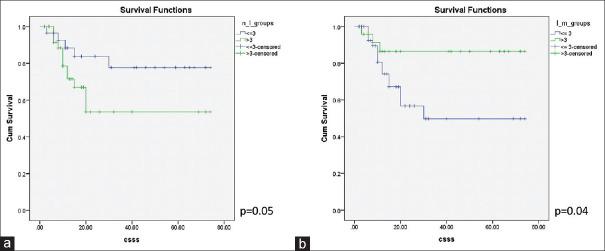
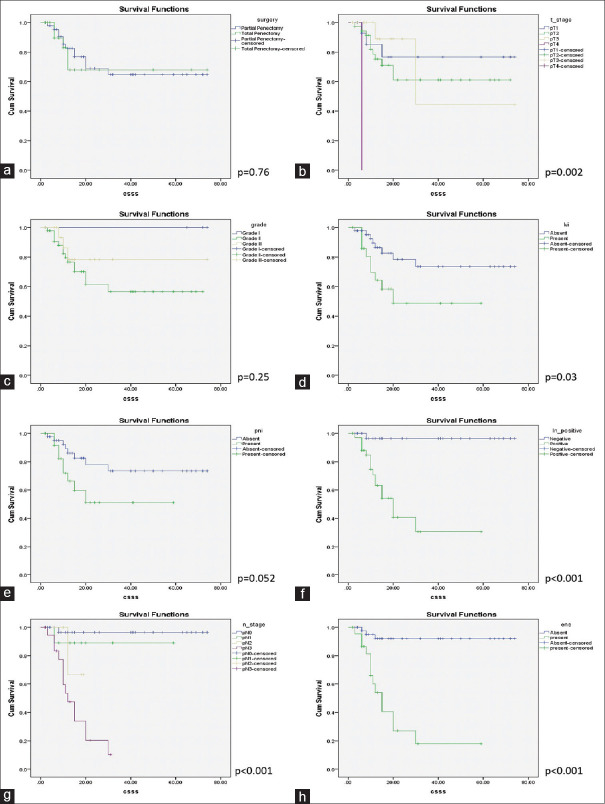
The Kaplan–Meier analysis revealed that NLR of >3 and the LMR of ≤3 indicated an inferior CSS with log-rank P values of 0.05 and 0.04, respectively [Figure 1]. It was also observed that advanced T stage was a predictor of inferior CSS (log-rank P = 0.002) and so were the presence of inguinal nodal involvement (log-rank P < 0.001), higher pathological nodal stage (log-rank P < 0.001), LVI (log-rank P = 0.03), and ENE (log-rank P < 0.001). The type of penile surgery, pathological grade, or the presence of PNI did not show any significant impact on CSS with P values of 0.76, 0.25, and 0.052, respectively [Figure 2]. On multivariate analysis, using all the variables that were significant on the univariate analysis, only T stage showed a statistically significant association with CSS (P = 0.02) [Table 1].
Figure 1.
The Kaplan–Meier curves showing cancer-specific survival stratified by (a) Neutrophil–lymphocyte ratio and (b) lymphocyte–monocyte ratio
Figure 2.
The Kaplan–Meier curves showing cancer-specific survival stratified by (a) type of surgery, (b) pathological T stage, (c) grade, (d) lymphovascular invasion, (e) perineural invasion, (f) nodal status, (g) pathological nodal stage, (h) extranodal extension
Analysis of pathological involvement of inguinal lymph nodes
On univariate analysis, the NLR >3 and LMR ≤3 were significantly associated with the presence of inguinal lymph node involvement (P = 0.001 and 0.026, respectively). In the pathological variables, the presence of LVI and PNI were significantly associated with the presence of inguinal node involvement having P < 0.001 and 0.02, respectively. However, on a multivariate analysis, only LVI showed a correlation with inguinal node involvement with P = 0.007 [Table 1].
DISCUSSION
The association of systemic inflammation and the development of cancers has been a hypothesis that is being proved in a number of solid organ malignancies. The Glasgow Inflammation Outcome Study which was conducted in a cohort of >200,000 participants found that the markers of inflammation, namely low albumin and high C-reactive protein, were elevated in patients with cancers as compared to controls.[10] These results encouraged the researchers to look out for new parameters that could indicate the presence of malignancy as well as help to prognosticate these patients.
In patients with carcinoma penis, the conventional pathological variables that determine the outcome have been the T stage, grade, LVI, N stage, etc.[2] In our study too, the T stage, LVI, pathological nodal status, pN stage, and ENE had a significant impact on the CSS, conforming to the literature. The success of inflammatory markers in other solid malignancies prompted their evaluation in patients with carcinoma penis. NLR has been found to be the most cost-effective marker and has been assessed in few studies. Kasuga et al. analyzed the CSS in 41 patients with carcinoma penis and found that on a univariate analysis, the patients with a NLR >2.82 had a poor CSS (P = 0.02), however, on a multivariate analysis, no factor was found to be independently predictive of the outcome.[6] Azizi et al. analyzed the NLR in 68 patients of carcinoma penis who had undergone inguinal lymph node dissection. They found that the median overall survival was significantly better in patients with a NLR <3 (30 vs. 158 months, P < 0.001).[5] Li et al., in the largest study published till date, analyzed 228 patients who had a bilateral inguinal node dissection for carcinoma penis. They found the NLR to be the single best predictor for the CSS ((hazard ratio: 2.131; P = 0.035).[8] In our study too, we found that NLR >3 predicted a poor CSS. However, the NLR lost its significance as an independent predictor on the multivariate analysis where only the T stage was found to be independently associated with poor CSS.
The LMR has been shown to have predictive value in cancer colon, breast, sarcoma, etc. Tan et al. conducted the first and the only study to analyze the LMR in 39 patients with carcinoma penis and found that a LMR <3.3 had a poor CSS (P < 0.022). Their multivariate analysis found that only the N stage was predictive of prognosis.[9] In our study too, the Kaplan–Meier curves showed that the patients with LMR ≤3 had an inferior CSS.
Inguinal lymph node dissection is associated with a significant morbidity, hence the importance to correctly identify the patients who have a nodal involvement is important. The pathological factors and clinical biomarkers have been described that might help the clinician in predicting the nodal involvement and thus planning the management accordingly.[3] NLR has also been found to have some role in predicting the pathological node involvement.[3,5,6] In our study, NLR >3 predicted a higher chance of inguinal node involvement. Interestingly, we also found that LMR <3 can also have a predictive value for involvement of inguinal nodes. This, to the best of our knowledge, has never been reported before in patients with carcinoma penis. However, it must be reiterated that on a multivariate analysis, only LVI was found to be associated with lymph node involvement. It is essential to emphasize that these ratios do show the potential to refine our existing predictive models for lymph node involvement in patients with carcinoma penis. There is a need of further research that can refine them and help in better identification and management of the inguinal nodes of patients of carcinoma penis.
Our study has certain strengths that are worth mentioning. First, the study population is homogeneous and of single ethnicity. Second, we excluded the patients who received neoadjuvant chemotherapy or radiotherapy. In the study by Azizi et al., 17.6% of the patients received some form of neoadjuvant treatment which might have affected the ratios.[5] Third, ours is a more contemporary cohort as compared to the two most recently published studies.[5,8] Fourth, classified all our patients according to the Eighth Edition of the American Joint Committee on Cancer TNM staging system for penile cancer rather than the older classification systems which were used in all the previous studies.[5,6,7,8,9] There have been significant changes in the eighth TNM classification system of carcinoma penis which can result in stage migration.[11] Hence, our results are more reflective of the current practice. Finally, to calculate the NLRs and LMRs, we used only that blood report which was performed within 2 weeks of surgery. The previous studies have either not defined this timing of the “preoperative” blood report used for the analysis or have taken a wide interval between the blood analysis and surgery.[5,6,9]
Our study is not without some limitations. First, this is a retrospective study and hence there is a possibility of inherent bias. Second, despite being one of the largest series, the number of patients in our study might still be considered to be low which can be attributed to the low incidence of the disease. We are optimistic that muti-institutional prospective studies would be able to address these limitations.
CONCLUSION
Our study demonstrates that in patients with squamous cell carcinoma of penis, NLR and LMR may help in predicting the CSS as well as the inguinal node involvement. It may be used as an additional guide, apart from the clinicopathological stage, to determine the prognosis of the patients with carcinoma penis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
s
REFERENCES
- 1.Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: Systematic review and meta-analysis. Rev Panam Salud Publica. 2017;41:e117. doi: 10.26633/RPSP.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah AA, Shah HA, Panjwani GN, Pandey BB, Shah N. Prognostic factors and 5-year survival of patients with carcinoma penis: Tertiary health center study. Indian J Cancer. 2016;53:309–12. doi: 10.4103/0019-509X.197729. [DOI] [PubMed] [Google Scholar]
- 3.Hu J, Cui Y, Liu P, Zhou X, Ren W, Chen J, et al. Predictors of inguinal lymph node metastasis in penile cancer patients: A meta-analysis of retrospective studies. Cancer Manag Res. 2019;11:6425–41. doi: 10.2147/CMAR.S206579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proctor MJ, Morrison DS, Talwar D, Balmer SM, O’Reilly DS, Foulis AK, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: A Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104:726–34. doi: 10.1038/sj.bjc.6606087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azizi M, Peyton CC, Boulware DC, Chipollini J, Juwono T, Pow-Sang JM, et al. Prognostic value of neutrophil-to-lymphocyte ratio in penile squamous cell carcinoma patients undergoing inguinal lymph node dissection. Eur Urol Focus. 2019;5:1085–90. doi: 10.1016/j.euf.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasuga J, Kawahara T, Takamoto D, Fukui S, Tokita T, Tadenuma T, et al. Increased neutrophil-to-lymphocyte ratio is associated with disease-specific mortality in patients with penile cancer. BMC Cancer. 2016;16:396. doi: 10.1186/s12885-016-2443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pond GR, Milowsky MI, Kolinsky MP, Eigl BJ, Necchi A, Harshman LC, et al. Concurrent chemoradiotherapy for men with locally advanced penile squamous cell carcinoma. Clin Genitourin Cancer. 2014;12:440–6. doi: 10.1016/j.clgc.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Li X, Zhang X, Chen P, Wang B, Chen X, et al. Prognostic significance of common preoperative laboratory variables in penile squamous cell carcinoma. Int J Urol. 2020;27:76–82. doi: 10.1111/iju.14137. [DOI] [PubMed] [Google Scholar]
- 9.Tan TW, Chia SJ, Chong KT. Management of penile cancer in a Singapore tertiary hospital. Arab J Urol. 2017;15:123–30. doi: 10.1016/j.aju.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proctor MJ, Talwar D, Balmar SM, O’Reilly DS, Foulis AK, Horgan PG, et al. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br J Cancer. 2010;103:870–6. doi: 10.1038/sj.bjc.6605855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornejo KM, Rice-Stitt T, Wu CL. Updates in staging and reporting of genitourinary malignancies. Arch Pathol Lab Med. 2020;144:305–19. doi: 10.5858/arpa.2019-0544-RA. [DOI] [PubMed] [Google Scholar]