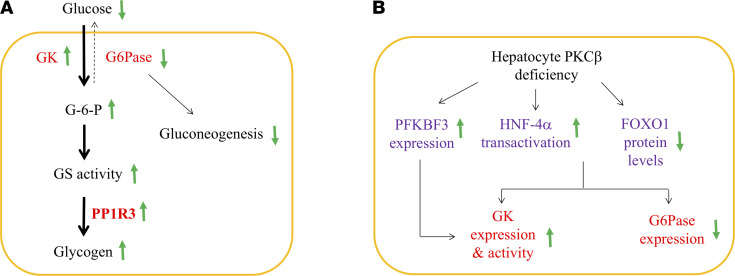
Figure 6. Proposed mechanisms by which hepatic PKCβ deficiency induces hepatic glycogen synthesis upon glucose overload, and the impact of PKCβ deficiency on transcription factors implicated in differential regulation of GK and G6Pase genes.
Our results link PKCβ with glycogen synthesis and extend our understanding of the underlying PKCβ-dependent transcriptional mechanisms for differential regulation of hepatic GK and G6Pase genes. (A) PKCβ deficiency enhances conversion of glucose to glycogen after glucose exposure by coordinating induction of GK expression and suppression of G6Pase expression, in combination with simultaneous dephosphorylation/activation of GS. These changes result in elevated GK/G6Pase ratio and intracellular G6P levels and promote glycogen synthesis and storage. In line with this model, GK has been suggested to have a close functional and regulatory association with glycogen synthesis through GS. PKCβ deficiency also induces expression of GK-binding protein PFK2/FBP2, a major allosteric regulator of its activity. Since G6Pase catalyzes the last step of gluconeogenesis, PKCβ deficiency can slightly reduce gluconeogenesis at the same time. The proposed mechanism has the potential to link diet-sensitive signaling kinase PKCβ with control of glycogenesis. (B) A model summarizing the orchestrated role of PKCβ in the differential regulation of hepatic GK and G6Pase genes in vivo. In this model, PKCβ contributes to the regulation of these genes through suppressing HNF-4α transactivation and enhancing FoxO1 protein stability. Thus, PKCβ deficiency enhances the ability of HNF-4α to transactivate and reduces the displacement of HNF-4α by FoxO1 from GK promoter, thereby resulting in induction of the GK promoter. At the same time, FoxO1 has also been reported to activate G6Pase expression through its synergistic interaction with HNF-4α; therefore, reduction of FoxO1 can also lead to decreased expression of the G6Pase gene. Up and down arrows indicate an increase or decrease, respectively, in protein level or activity in the liver.