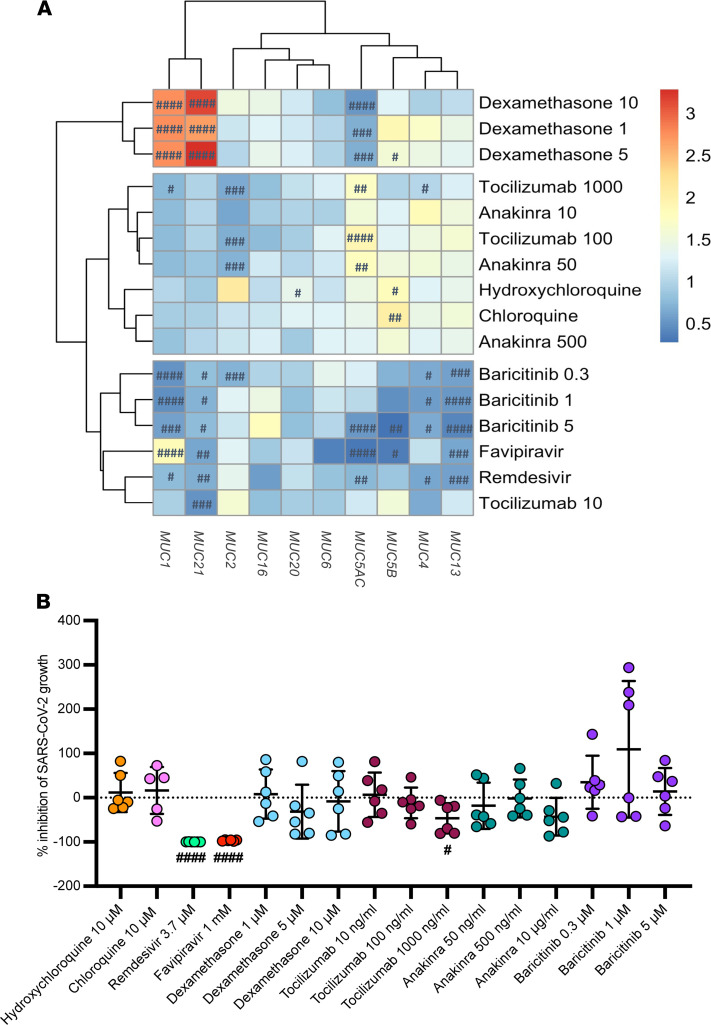
Figure 8. Ability of FDA-approved drugs for COVID-19 to reduce mucin overexpression in SARS-CoV-2–infected pulmonary epithelial cells.
(A) Heatmap visualization of the relative mRNA expression data of MUC1, MUC2, MUC4, MUC5AC, MUC5B, MUC13, MUC16, MUC20, and MUC21 in pulmonary (Calu3) epithelial cells infected with SARS-CoV-2 at 0.1 MOI for 2 hours and thereafter treated with a COVID-19 drug at different concentrations for 48 hours (n = 6). These include remdesivir (3.7 μM); favipiravir (1 mM); (hydroxy)chloroquine (10 μM); dexamethasone (1, 5, and 10 μM); tocilizumab (10, 100, and 1000 ng/mL); anakinra (50 and 500 ng/mL, 10 μg/mL), and baricitinib (0.3, 1, 5 μM). Significant differences between treated and untreated cells upon SARS-CoV-2 infection are indicated by #P < 0.05; ##P < 0.01; ###P < 0·001; ####P < 0.0001. One-way ANOVA, Tukey’s post hoc multiple-comparison test. (B) Percentage of inhibition of SARS-CoV-2 growth in Calu3 cells upon treatment with a COVID-19 drug (n = 6). Data are presented as mean ± SEM. Significant differences between treated and untreated cells upon SARS-CoV-2 infection are indicated by #P < 0.05; ##P < 0.01; ###P < 0·001; ####P < 0.0001. One-way ANOVA, Tukey’s post hoc multiple-comparison test.