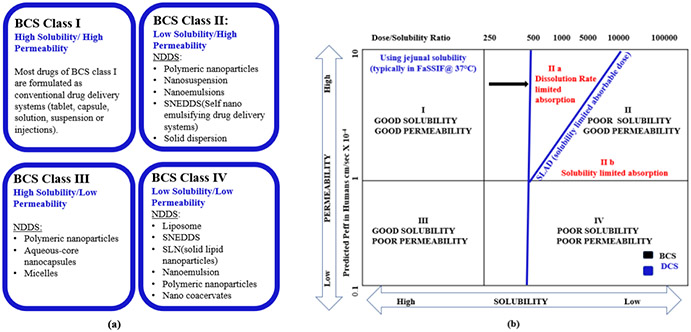
FIGURE 3.
Nano drug delivery systems (NDDS) for various BCS class. Class I drugs are straightforward to develop. NDDS offer promising drug delivery solutions for potential drug candidates belonging to BCS class II, III and IV by overcoming poor solubility/permeability issues. (b) DCS classification system. SLAD marks the boundary between DCS class IIa and IIb drugs. Below SLAD all the doses could be dissolved, and above SLAD only a fraction of dose is dissolved (fraction dissolved decreases with increasing dose). DCS Class IIa drugs show ‘dissolution-rate limited’ absorption. Simple particle size reduction can suitably formulate such drugs. Therefore, particle size (in contrast to dose/solubility ratio of BCS classification) can better predict the extent of absorption. DCS Class IIb drugs have ‘solubility-limited’ absorption, and they present major formulation challenges (bioavailability depends on gastric pH and intestinal precipitation can occur). These drugs remain incompletely absorbed unless suitably solubilized (e.g., lipid-based formulation or amorphous solid dispersion)