Figure 6: The dynamics of the B[a]P rings intercalated into the helix shows that the K56ac causes the B[a]P ring system to be more dynamic than in the unacetylated K56.
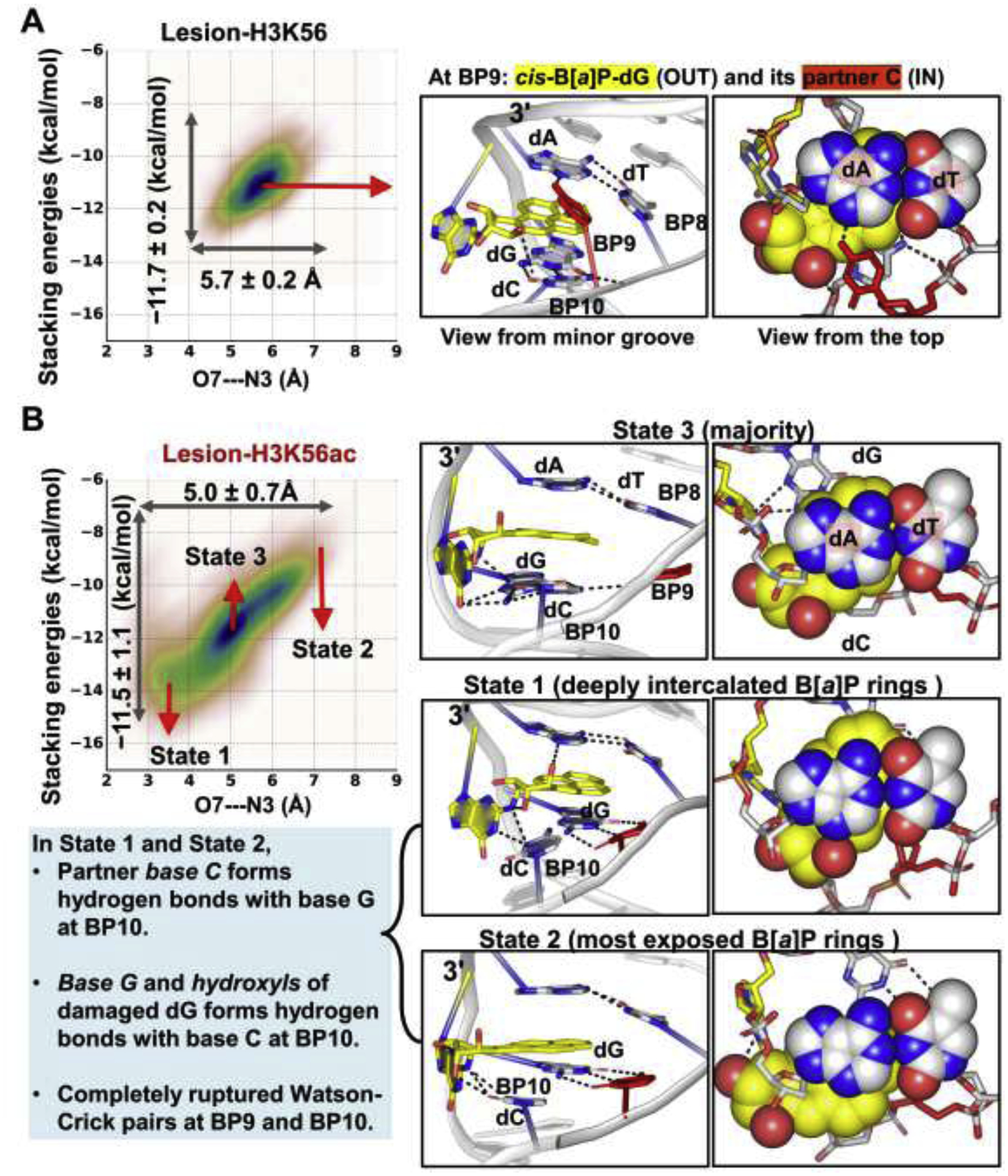
The O7—N3 distance and the stacking interactions of the B[a]P rings with the bases at BP8 are plotted against each other for the lesion-containing NCPs. Mean values and standard deviations are given; the larger range and standard deviations reflect the larger span that the B[a]P rings sample and the greater dynamics of the B[a]P rings. The larger ranges and standard deviations are revealed in the Lesion-H3K56ac NCP; when H3 K56 is acetylated, the intercalated B[a]P rings sample a larger span with enhanced dynamics compared to the unacetylated K56.
(A) In Lesion-H3K56, the B[a]P rings stack stably with the adjacent bases at BP8 (Figure S5).
(B) In Lesion-H3K56ac, the intercalated B[a]P ring system stacks dynamically with the adjacent bases between two extreme states (State 1 and State 2). In State 1, the hydroxyl group in the B[a]P rings forms a hydrogen bond with the N3 atom of the A base at BP8 (O7—N3 distance), resulting in a deeply- intercalated and least exposed B[a]P ring system. In State 2, the hydroxyls of the B[a]P rings and A base are most distanced, resulting in the least intercalated and most exposed B[a]P rings. Notably, in these two extreme states, the partner base C forms three hydrogen bonds with the base G (5′-side to the lesion) at BP10, and the damaged base G and hydroxyls of the lesion interact with base C at BP10, leading to a completely ruptured Watson-Crick base pair at BP10 and a partially ruptured WC pair at BP11 (Figure S7D); this is likely due to the neighboring sequence context. The majority conformation of the B[a]P rings is in State 3 where the Watson-Crick pair at BP10 and BP11 present as in the unacetylated K56 case. Structures indicated in the correlation plot are shown in the right panel. The 3-mer duplex GG*A with lesion (G* = cis-B[a]P-dG) at the center are rendered as sticks.
