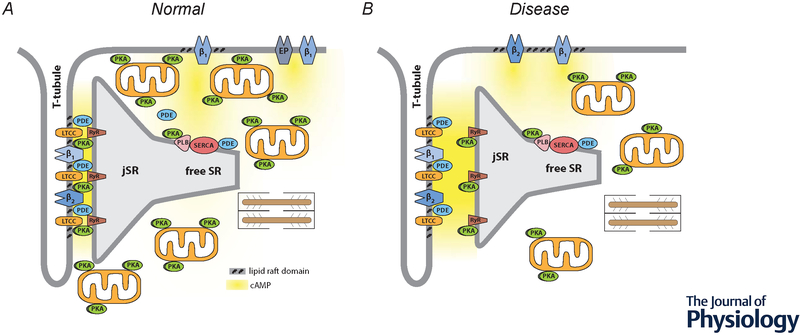
Figure 1.
Compartmentation of receptor-dependent cAMP signaling in adult ventricular myocytes. A, Under normal conditions, dyadic clefts are formed by tight junctions between the plasma membrane of t-tubules and the junctional sarcoplasmic reticulum (jSR). This is where β1 and β2ARs found in the plasma membrane of t-tubules are believed to be part of caveolar signaling complexes that include L-type Ca2+ channels (LTCCs), which are in close proximity to ryanodine receptors (RyRs) found in the jSR. E-type prostaglandin (EP) receptors, as well as some β1ARs, are excluded from of caveolar signaling complexes and t-tubule membranes. Phospholamban (PLN) and the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) are found outside of dyadic clefts in the free SR. Under normal conditions, β1AR production of cAMP leads to protein kinase A (PKA)-dependent phosphorylation of LTCCs, RyRs, and PLN. β2ARs lead to phosphorylation of LTCCs, but not RyRs or PLN. EP receptors do not regulate any of these effectors. Strategically placed phosphodiesterase (PDE) activity plays a critical role in cAMP compartmentation. Restricted spaces such as those created by dyadic clefts and tight mitochondrial packing as well as buffering by PKA anchored to the outer membrane of mitochondrial and other structures may also contribute to this behavior. B, Disease states, such as heart failure, are associated with changes in factors believed to contribute to compartmentalized cAMP responses. These changes include: disruption of dyadic clefts; redistribution of β2ARs from t-tubules to the peripheral sarcolemma; loss of AKAPs anchoring PKA to mitochondria; disruption of mitochondrial organization; and loss of PDE activity in some locations.