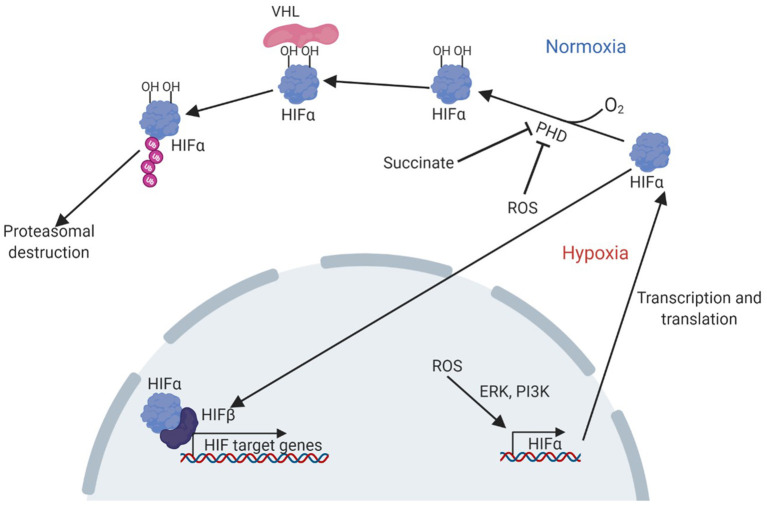
Figure 1.
Pathway of HIF activation in hypoxia. Adapted from Lee et al. (7). Under normoxic conditions, PHD enzymes hydroxylate proline residues in the HIFα subunit, in an oxygen-dependent manner. The hydroxylated residues are bound by VHL, which ubiquitinates HIFα allowing recognition and destruction of HIFα by the proteasome. Under hypoxic conditions, hydroxylation cannot occur and HIFα can instead translocate to the nucleus, bind HIFβ and other cofactors to activate transcription of target genes. HIF accumulation can also result from PHD inhibition by succinate or ROS, or by increased transcription and translation due to a ROS induced, ERK and PI3K mediated pathway. ERK, extracellular-signal related kinase; HIF, hypoxia-inducible factor; PHD, prolyl hydroxylase domain proteins; PI3K, phosphoinositide 3-kinase; VHL, Von Hippel Lindau protein.