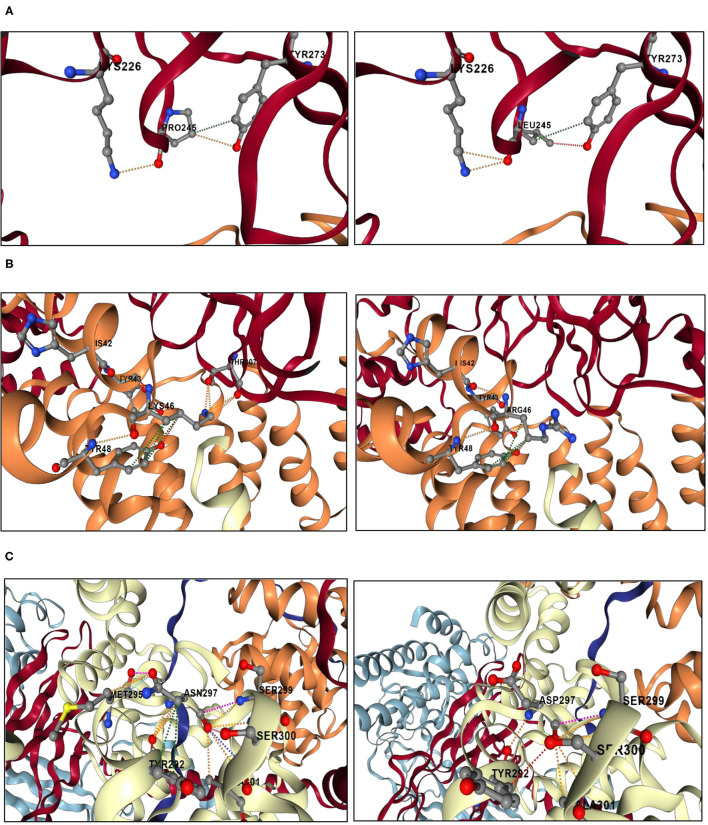
Figure 3.
Structural analyses results of the genetic variants located in the BBSome. The analyses were performed using DynaMut2 server. (A) Structural analysis of the BBS1 p.Pro245Leu mutation. A fraction of BBS1 subunit is shown in red. A fraction of BBS4 is shown in orange. The left panel shows a close-up view of the interactions between Pro245 and the surrounding residues. Orange dashes represent polar bonds between Pro245 and Tyr273 and Lys226. Green dashes represent hydrophobic interaction bond between Pro245 and Tyr273. Oxygen atoms are colored in red, nitrogen atoms are colored in blue, and carbon atoms are colored in gray. The right panel shows a close-up view of the interactions between Leu245 and the surrounding residues. The mutant residue Leu245 replaces the polar bonds originally made by the wild-type residue Pro245 with the Tyr273 by weaker hydrogen bonds (shown in red dashes). (B) Structural analysis of the BBS4 p.Lys46Arg mutation. A fraction of BBS1 subunit is shown in red. A fraction of BBS4 is shown in orange. A fraction of TTC8 is shown in white. The left panel shows a close-up view of the interactions between Lys46 and the surrounding residues. Orange dashes represent polar bonds between Lys46, Tyr43, and Tyr48 in the BBS4 subunit, as well as between the Lys46 and the Thr308 in the BBS1 subunit. Green dashes represent hydrophobic interactions between Lys46 and Tyr48. Oxygen atoms are colored in red, nitrogen atoms are colored in blue, and carbon atoms are colored in gray. The right panel shows a close-up view of the interactions between Arg46 and the surrounding residues. The mutant residue Arg46 may not be able to make polar contacts with the residue Thr307 of the BBS1 subunit. (C) Structural analysis of the TTC8 p.Asn297Asp mutation. A fraction of TTC8 subunit is shown in white. The left panel shows a close-up view of the interactions between Asn297 and the surrounding residues. Orange dashes represent polar bonds between Asn297, Met295, Tyr292, Ala301, Ser300, and Ser299. Sky blue dashes represent Van der Waals bonds between Asn297 and Met295. Green dashes represent hydrophobic interactions between Asn297and Tyr292. Oxygen atoms are colored in red, nitrogen atoms are colored in blue, and carbon atoms are colored in gray. The right panel shows a close-up view of the interactions between Asp297 and the surrounding residues. The mutant residue Asp297 disrupts interactions initially made by the wild-type residue Asn297 with the residue Met295.