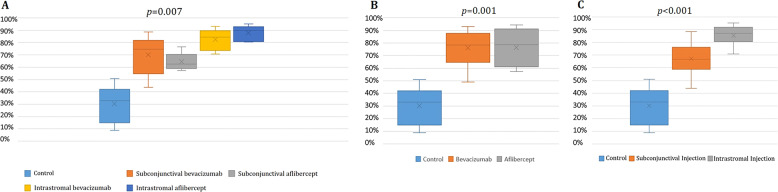
Fig. 3. Percent reductions in CoNV areas in different treatment groups.
Percent reductions in CoNV area were 30.3% in the control group, 69.9% in the subconjuctival bevacizumab group, 64.5% in the subconjunctival aflibercept group, 82.5% in the intrastromal bevacizumab group and 88.1% in the intrastromal aflibercept group (p* = 0.007). In pairwise comparison of each group with the control group, p# = 0.010 for subconjunctival bevacizumab vs. control, p# = 0.005 for subconjuctival aflibercept vs. control, p# = 0.003 for intrastromal bevacizumab vs. control, and p# < 0.001 for intrastromal aflibercept vs. control. A Percent reductions in CoNV area were 76.2% in the bevacizumab group, and 76.3% in the aflibercept group, regardless of the administration route (p* = 0.001), p# = 0.002 for bevacizumab vs. control, p# = 0.001 for aflibercept vs. control, p# = 0.609 for bevacizumab vs. aflibercept). B Percent reductions in CoNV area were 67.2% in the subconjunctival group, and 85.3% in the intrastromal group, regardless of the type of anti-VEGF. (p* < 0.001). In pairwise comparison, p# = 0.005 for subconjunctival injection vs. control, p# < 0.001 for intrastromal injection vs. control, p# = 0.039 for subconjunctival injection vs. aflibercept injection). *Kruskal–Wallis test, #Mann–Whitney U test.