Abstract
Context:
Early integrated palliative care improves quality of life, but palliative care programs are underutilized. Psychoeducational interventions explaining palliative care may increase patients' readiness for palliative care.
Objectives:
To (1) collaborate with stakeholders to develop the EMPOWER 2 intervention explaining palliative care, (2) examine acceptability, (3) evaluate feasibility and preliminary efficacy.
Methods:
The research was conducted at a North American cancer center and involved 21 stakeholders and 10 patient-participants. Investigators and stakeholders iteratively developed the intervention. Stakeholders rated acceptability of the final intervention. Investigators implemented a pre-post trial to examine the feasibility of recruiting 10 patients with metastatic cancer within one month and with a ≥50% consent rate. Preliminary efficacy outcomes were changes in palliative care knowledge and attitudes.
Results:
Using feedback from four stakeholder meetings, we developed a multimedia intervention tailored to three levels of health-literacy. The intervention provides knowledge and reassurance about the purpose and nature of palliative care, addressing cognitive and emotional barriers to utilization. Stakeholders rated the intervention and design process highly acceptable (3.78/4.00). The pilot met a priori feasibility criteria (10 patients enrolled in 14 days; 83.3% consent rate). The intervention increased palliative care knowledge by 83.1% and improved attitudes by 18.9 points on a 0-51 scale (ps<.00001).
Conclusions:
This formative research outlines the development of a psychoeducational intervention about palliative care. The intervention is acceptable, feasible, and demonstrated promising pilot test results. This study will guide clinical teams in improving patients’ readiness for palliative care and inform the forthcoming EMPOWER 3 randomized clinical trial.
Keywords: Palliative care, Neoplasms, Patient education, Attitude, Decision support techniques, stakeholder participation, professional practice gaps, information dissemination
Palliative care faces a fundamental research-practice gap. Namely, high-quality medical evidence demonstrates the efficacy of specialty palliative care programs (1-5), but these programs are poorly utilized (6-10). Given that oncology care is highly guideline-driven and many patients experience burdensome physical symptoms (60-90%) and emotional distress (25-40%) (11-14), specialty palliative care programs are integrating their services with oncology care in many healthcare systems (7, 15). According to the early integrated palliative care model (10, 16-18), patients should begin participating in outpatient palliative care early after the diagnosis of metastatic disease and alongside cancer treatments. Four meta-analyses of randomized clinical trials (RCTs) show that early integrated palliative care reduces the physical and emotional burden of metastatic cancer (1-5) and maintains (1) or slightly prolongs (3-5) survival duration. Consensus guidelines now advocate for early integrated palliative oncology care (19-21). Nonetheless, utilization is often delayed until patients are near death, and most never receive palliative care (6-10). Therefore, healthcare systems that seek to provide early integrated palliative oncology care need to better support patients’ decisions to utilize it.
The Empowerment Theory of Palliative Care (22) suggests that psychoeducational interventions can help increase readiness to utilize early integrated palliative care. Grounded in decades of decision science, this theory emphasizes that the dual processes of cognition and emotion drive interest in palliative care. By providing patients with vital knowledge (cognitive pathway) and increased motivation (emotional pathway), patients feel empowered to utilize timely palliative care referrals. Cognitively, 70-80% of adults report not knowing what palliative care is (23, 24). Emotionally, patients equate palliative care utilization with dying, giving up, welcoming the grim reaper, or being on the wrong side of “death panels” (22, 25-29). These cognitive and emotional barriers govern patient reluctance to utilize early integrated palliative care.
Meta-analyses document the benefits of psychoeducational interventions in changing knowledge, motivation, health behaviors, and utilization patterns, but effects vary widely (30, 31). Four RCTs on palliative care psychoeducation stand out. The EMPOWER RCT (22) found that a webpage summarizing the Temel (32) study improved attitudes toward early integrated palliative care among 598 patients with heterogeneous cancers. A second RCT (33) found that video and text-based psychoeducational interventions increased knowledge of palliative care in a non-clinical sample of 152 adults. A third RCT (34) examining a video-based intervention found no change in palliative care knowledge among 111 women with gynecologic cancers. Finally, the PCforME RCT (35) found no benefit of a video-based palliative care educational platform on knowledge of palliative care among 80 patients meeting palliative care referral criteria. Given these variable results, we deduced that the intervention development process requires careful consideration.
In the current study, EMPOWER 2, we built on findings from the EMPOWER RCT described above. Specifically, the present study aimed to develop an intervention to explain palliative care to patients and families and to evaluate its acceptability, feasibility of implementation, and initial evidence of efficacy. In the prior EMPOWER study (22), the authors tested a public internet-based intervention. However, palliative care programs vary by healthcare system (36-38). In the present EMPOWER 2 study, we developed an intervention that was tailored to local palliative care services and intended to be optimally informative and motivational. For example, the EMPOWER 2 intervention was video-based, tailored to individuals’ health literacy, and provided a comprehensive explanation of palliative care that dispelled common misconceptions associated with negative perceptions and avoidance of palliative care. If successful, this research can guide other healthcare systems in improving readiness for their palliative care programs.
Methods
Intervention Design
Theoretical Framework.
The intervention design was grounded in the Empowerment Theory of Palliative Care (22), which indicates that cognitive and emotional processes drive utilization of palliative care. This theory emphasizes that psychoeducational interventions should increase knowledge (cognitive pathway) and motivation (emotional pathway) to improve patient readiness. Therefore, the investigators designed the intervention to optimize cognitive and emotional benefits for patients (Table 1).
Table 1.
Theoretical Basis: Intervention Targets Cognitive and Emotional Pathways to Increase Readiness to Utilize Palliative Care Programs
| Cognitive Pathway | Emotional Pathway |
|---|---|
|
|
Design Process.
The design process was informed by principles of patient-centered research aimed at helping patients make informed decisions about their healthcare. These included guidelines from multiple sources, such as the Patient-Centered Outcomes Research Institute (PCORI) (39) and the International Patient Decision Aids Standards (IPDAS) collaborative (40). The investigators used a structured process to outline, develop, revise, evaluate, and scale up the intervention (Table 2). Given that stakeholder engagement can increase the impact of interventions (41, 42), we recruited a stakeholder advisory board using the regional Health in Our Hands stakeholder recruitment network. The stakeholder advisory board was comprised of patients with metastatic cancer, family members, and oncology clinicians helped design the intervention (Table 2, steps 3, 6, 8, and 10). They participated in 4 advisory meetings from January 2017 through December 2018. Stakeholders provided feedback on the intervention format and scope (meeting 1), guided iterative improvements to the intervention script and prototype (meetings 2-3), and evaluated the acceptability of the final intervention (meeting 4). The meetings were highly collaborative, and used reflective listening, amplification, clarifying questions, and discussion-based brainstorming to build consensus (43).
Table 2.
Intervention Development Process
| Preparation |
| 1. Define scope and purpose 2. Recruit stakeholders 3. Gain preliminary stakeholder input 4. Conduct meta-analysis |
| Intervention Design |
| 5. Design intervention prototype 6. Conduct stakeholder alpha testing 7. Design formal intervention 8. Conduct stakeholder beta testing 9. Finalize intervention |
| Evaluation |
| 10. Evaluate intervention acceptability 11. Estimate intervention design costs 12. Evaluate implementation feasibility 13. Evaluate initial evidence of efficacy |
| Scale Up |
| 14. Secure funding for RCT |
Pilot Testing
We piloted the intervention at the Tulane Cancer Center in September 2019. Inclusion criteria were being ≥18 years old and having metastatic cancer. Patients were excluded if they had a prior palliative care visit. Oncologists confirmed eligibility and informed patients of the study, who were invited to participate before or after an oncology visit. The study used a pre-post design. Participants completed a baseline survey, watched the intervention once from start to finish, and then completed a post-intervention survey immediately afterward. The baseline survey assessed demographics, while both the baseline and post-intervention surveys assessed palliative care knowledge and attitudes (see Outcome Evaluation). Surveys were administered orally to accommodate limited literacy and vision. Family members who were present at the oncology visit were encouraged to attend. The Institutional Review Board approved all procedures (#2018-1253).
Outcome Evaluation
Acceptability.
Stakeholders (N=21) evaluated the acceptability of the intervention. Internal stakeholders (n=7) on the advisory board helped design the intervention and rated the acceptability of the design process and final intervention. External stakeholders (n=14) did not design the intervention and solely rated the acceptability of the final intervention. Stakeholders primarily identified as patients (n=7, [3 internal]), caregivers (n=6, [2 internal]), or clinician-scientists (n=8, [2 internal]). Both internal and external perspectives are useful (40). Stakeholders completed a 6-item version of the IPDAS instrument (44). From 1 (strongly disagree) to 4 (strongly agree), they rated how well the video described aspects of palliative care (items 1-4), and internal stakeholders also rated whether the development process engaged patients/family members (item 5) and clinicians (item 6). Mean ratings of 3 (agree) or higher indicated acceptability.
Feasibility of Implementation.
In pilot testing, we determined a priori that a sample size of 10 would be sufficient for estimating whether we could recruit enough eligible patients on a reasonable timeline to meet our target sample size for a follow-up RCT. We defined a feasible implementation as one which met both of the following criteria: 1) >50% of eligible patients approached must consent to and complete the study, and 2) the accrual time needed for 10 eligible patients to complete the study must be within one month of commencing data collection. If met, these criteria would document the ability of the investigators to meet accrual timelines in a larger-scale RCT, where approximately four patients would need to be enrolled per month.
Preliminary Efficacy.
In the pilot study, the two efficacy outcomes were palliative care knowledge and attitudes. Knowledge was assessed using the Palliative Care Knowledge Scale (PaCKS) (45). This 13-item measure allows participants to respond “true,” “false,” or “don’t know,” to statements reflecting facts and misconceptions about palliative care (e.g., “Palliative care is exclusively for people who are in the last 6 months of life.” [false]). Responses were scored to indicate the proportion correct. Attitudes were measured using the Palliative Care Attitude Scale (PCAS-9) (26), which has three subscales assessing fear of palliative care (emotional subscale), perceived benefits of palliative care (cognitive subscale), and willingness to accept referrals (behavioral subscale). The emotional subscale is reverse-coded so that higher scores indicate greater comfort with palliative care. The three subscales are summed to provide a total score from 0-51, with higher scores indicating more favorable attitudes toward palliative care. Repeated-measures t-tests were used to examine pre-post changes in outcomes, using an alpha-level of .05, confidence intervals, and Cohen’s d (46) to characterize effect sizes (small effect: d=0.20; medium effect: d=0.50; large effect: d=0.80).
Results
Stakeholder Meetings
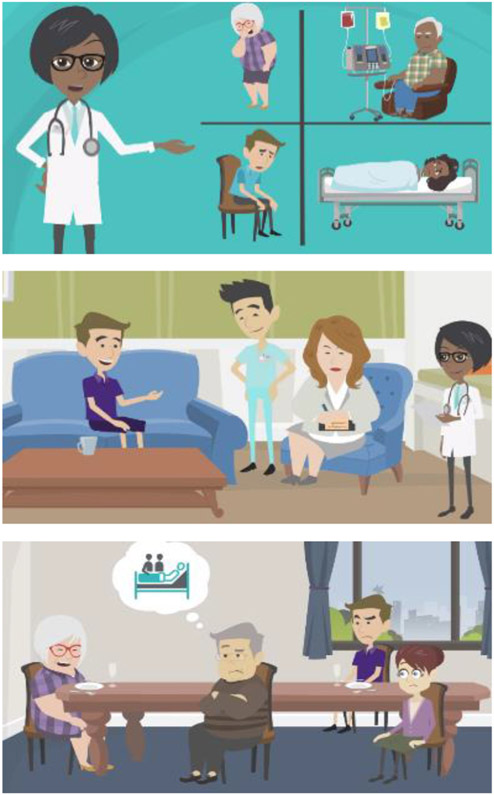
The first stakeholder meeting built rapport, informed stakeholders about palliative care and the research plan, and solicited feedback on the anticipated intervention content. Stakeholders provided big-picture feedback: make the intervention is accessible and relatable, use video, and tailor it to the educational background of each patient. Accordingly, the investigators developed the intervention using a professional multimedia animation platform called Vyond. The platform allows investigators to compose dynamic video scenes (Figure 1) with audio voiceover and text. The investigators drafted scripts for three versions of the video – basic, intermediate, and advanced – that varied in literacy level and complexity. The videos span 9 chapters (Table 3) and are brief (7-13 minutes).
Figure 1.
Intervention Video Sample Scenes, Developed using Vyond Platform
Table 3.
Chapters of the Psychoeducational Video Describing Palliative Care Programs to Patients and Families
| Chapter Title | Description |
|---|---|
| 1. Welcome | Describes the overall purpose of the video |
| 2. Palliative Care Overview | Provides a general introduction to palliative care |
| 3. Palliative Care Team | Describes concretely who is on a palliative care team |
| 4. Typical Visit | Highlights what palliative care visits look like |
| 5. Who Should Use Palliative Care | Reviews the types of patients who are and are not appropriate for palliative care programs |
| 6. Caregiver Involvement | Introduces ways caregivers can be involved in palliative care |
| 7. Evidence Base | Reviews the scientific evidence base that supports palliative care |
| 8. Responding to Misconceptions | Debunks common misconceptions patients may have about palliative care and offers example responses to others’ misconceptions |
| 9. Local Resources | Provides patients with information to access local palliative care resources |
Note. In a follow-up RCT, the investigators have added a brief 10th chapter defining how local palliative care programs have adapted service delivery in response to the COVID-19 pandemic.
In the second and third stakeholder meetings, stakeholders engaged in alpha testing and beta testing to iteratively improve the intervention. The stakeholders reviewed scripts for the basic, intermediate, and advanced versions of the intervention and reviewed sample clips from working drafts of videos. They provided detailed feedback on dialogue, lines of reasoning, and visual elements. The stakeholders also provided big-picture feedback, for example, recommending the inclusion of a chapter on supporting family caregivers (Table 3, Chapter 6). They reinforced several elements of the intervention: the professionalism in communicating sensitive topics, the relatability of the scenes depicted in patients’ homes and healthcare settings, and the incorporation of diverse characters. The stakeholders endorsed the intervention’s use of two-sided arguments (i.e., indicating pros and cons, who should and should not utilize, and how palliative care is similar to and different from hospice). Stakeholders also suggested we post the videos online and provide handouts so patients and families could revisit the material at home.
In the fourth meeting, stakeholders reviewed the final intervention, rated its acceptability (see Acceptability), and provided open-ended summative feedback. Stakeholders emphasized that they appreciated the power of the video, its ability to transform fear into comfort, the facts included about palliative care, the video’s cultural sensitivity to diverse groups, and the usefulness of incorporating local information. Stakeholders helped the investigators to identify how patients should be routed to the appropriate intervention version (i.e., basic, intermediate, or advanced) based on educational background and health literacy levels (Appendix Figure A1).
Description of the Intervention
The intervention targets the cognitive and emotional pathways that govern readiness to utilize palliative care (Table 1), encompasses 9 chapters (Table 3), and is tailored to three levels of complexity for patients with varying educational backgrounds and levels of health literacy (Appendix Figure A1). In terms of the cognitive pathway, the intervention provides information grounded in meta-analyses, consensus statements, and guidelines for decision interventions. In terms of the emotional pathway, the intervention is grounded in psychology and marketing principles and mentions emotions >15 times. The basic version is appropriate for individuals with limited to no literacy and limited health literacy. It avoids text and numbers, describes concepts concretely, and has a low vocabulary level (Flesch-Kincaid grade level of 4.4). In contrast, the advanced version has plenty of extra detail, nuance, and a higher vocabulary level (Flesch-Kincaid grade level of 9.8). The full video ranges from 7m 21s (1,100 words) for the basic version to 12m 38s (1,800 words) for the advanced version (scripts and video design cost estimates are available upon request). Characters in the video are diverse with respect to age, gender, race, functional status, and family composition. Similarly, the video includes voiceover from four professional voice actors: an African American woman, an African American man, a White woman, and a White man. These subgroups represent >90% of our patient population. The videos can be administered on a tablet computer during clinic visits, and patients and families can watch the video on their home computer or phone.
The intervention also incorporates six supplemental handouts recommended by stakeholders. These include a business card with a link and passcode to view the video online at home, a one-sheet video summary, a question-prompt list for discussing palliative care with oncology clinicians, a local palliative care program brochure, an American Society of Clinical Oncology (ASCO) palliative care booklet (47), and a Center to Advance Palliative Care (CAPC) flyer for patients and families (48). The handouts reinforce the video by providing consistent information across multiple authoritative sources and allowing patients and families to revisit content at home.
Acceptability
Stakeholders (N=21) evaluated the intervention favorably with a mean rating of 3.78 (SD=0.44) across items (Table 4). Reviewing each rating, the intervention successfully described available options, described pros and cons, identified psychological and physical benefits, and meaningfully engaged stakeholders in the development process. The ratings met a priori criteria for acceptability.
Table 4.
Stakeholder Ratings of the Acceptability of the Final Intervention
| Statement | M | SD |
|---|---|---|
| 1. The video describes the options available related to palliative care. | 3.76 | 0.44 |
| 2. The video makes it possible to understand the positive and negative features of palliative care. | 3.43 | 0.60 |
| 3. The video describes palliative care in a way that allows people to understand that it can help physically. | 3.90 | 0.30 |
| 4. The video describes palliative care in a way that allows people to understand that it can help psychologically. | 3.43 | 0.60 |
| 5. Video development involved finding out what patients/families wanted the video to say about palliative care. * | 3.83 | 0.41 |
| 6. Video development involved finding out what cancer clinicians wanted the video to say about palliative care. * | 4.00 | 0.00 |
| Average | 3.78 | 0.44 |
N = 21 stakeholders: 7 internal who helped design video, 14 external stakeholders. Stakeholders rated each response on a scale from 1 (strongly disagree) to 4 (strongly agree). Stakeholders primarily identified as patients (n=7, [3 internal]), caregivers (n=6, [2 internal]), or clinician-scientists (n=8, [2 internal]).
External stakeholders did not rate items 5-6
Feasibility of Implementation
From September 4 to September 17 of 2019, the investigators approached 12 eligible patients to participate in the pilot study. Of these patients, 10 (83.3%) consented and completed the study, and 2 (16.7%) declined because of childcare or transportation concerns. Diagnoses included female genital (n=4), prostate (n=2), urinary tract (n=2), gastrointestinal tract (n=1), and lung (n=1) cancer. The sample was 60% female and ranged from 28-88 years old (Mdn=60). Participants were representative of the local clinical population with respect to race/ethnicity (60% White, 40% African American, 20% Latino/a) and education level (40% with a high school diploma or less). The proportion enrolled and accrual rate met a priori criteria for feasibility. Each participant successfully watched the entire video.
Preliminary Efficacy
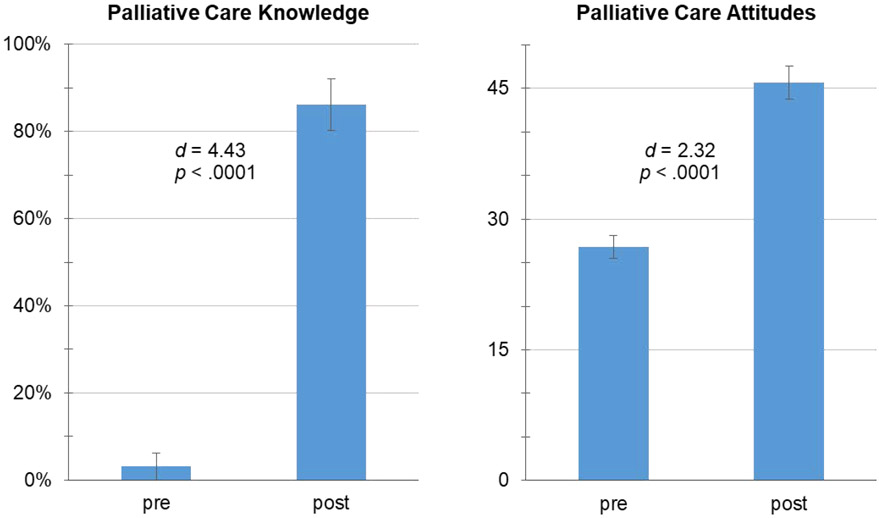
The intervention showed preliminary evidence of improving participants’ knowledge and attitudes about palliative care (Figure 2). Knowledge improved 83.1%, increasing from 3.1% correct pre-intervention to 86.2% correct post-intervention, t=13.99, d=4.43, p<.0001. Attitudes toward palliative care improved by 18.9 points, increasing from 26.8 (SD=4.24) at pre-intervention to 45.7 (SD=5.96) post-intervention (t=7.34, d=2.32, p<.0001). Attitude changes included reduced fear of palliative care (emotional subscale: t=6.79, d=2.15, p<.0001), greater belief in the efficacy of palliative care (cognitive subscale: t=6.61, d=2.09, p<.0001), and stronger intentions to utilize palliative care (behavioral subscale: t=6.68, d=2.11, p<.0001). All observed effects of the intervention exceeded those traditionally characterized as large (i.e., d=0.80).
Figure 2.
Pre-intervention to Post-Intervention Improvement in Knowledge of Palliative Care and Attitudes toward Palliative Care in a Pilot Sample of Patients (N=10) with Metastatic Cancer, Mean ± Standard Error
Discussion
The EMPOWER 2 study successfully developed a psychoeducational intervention about palliative care that was guided by theory and stakeholder engagement. Stakeholders comprised of patients, families, and oncology clinicians favorably evaluated the acceptability of the intervention. Moreover, >80% of patients approached about the study agreed to participate, and the investigators outpaced their targeted accrual rate, demonstrating the intervention’s feasibility of implementation. A pre-post trial conducted in a diverse group of patients with metastatic cancer produced encouraging-but-preliminary intervention effect sizes for increasing palliative care knowledge and attitudes, which were approximately 3-5 times the magnitude of what is traditionally defined as a “large” effect (46). These promising preliminary findings indicate the need for a large RCT aimed at testing the intervention’s efficacy for improving knowledge, attitudes, and utilization of early integrated palliative care.
Our results suggest that careful attention to the process of designing psychoeducational interventions about palliative care is instrumental to their success. Foremost, the intervention was grounded firmly in theory, emphasizing the cognitive and emotional pathways underlying palliative care readiness. Rather than simply summarizing cold meta-analytic evidence (1-5), the intervention leveraged psychology and marketing principles to engage, persuade, and empower. Second, stakeholders contributed substantially to the intervention design, increasing the likelihood it would be informative, relatable, professional, culturally responsive, and motivating. Stakeholder engagement requires an iterative time-intensive process, additional funds, and the willingness of investigators to listen to critical feedback. Too often, these barriers diminish interest in stakeholder-informed research (49), even though these processes increase intervention effectiveness (41, 42). In sum, patients’ palliative care knowledge and attitudes appear highly malleable when approached meticulously.
This research will help other investigators to implement psychoeducational interventions successfully. The investigators will share the EMPOWER 2 intervention script and videos with other teams at no cost upon completing a resource-sharing agreement. However, we also carefully delineated the intervention design process, stakeholder meetings, the resulting intervention, and design cost estimates to guide other researchers in developing localized interventions. The availability and key elements (50) of palliative care programs vary by healthcare system (36-38), and the rationale for seeking palliative care depends on the diagnoses, demographics, and unmet needs of local patient populations. “Glocalization,” thus, seems a reasonable strategy – acting globally to educate patients about palliative care while tailoring interventions to local practices. Although we focused on palliative care, the study offers an illustrative example for investigators seeking to develop psychoeducational interventions for other novel or underutilized healthcare programs.
Strengths and Weaknesses
A major strength was the aim of this investigation – addressing the important topic of palliative care research-practice gaps. We also meaningfully engaged stakeholders and included preliminary efficacy data from a racially and educationally diverse sample of adults with metastatic cancer. However, there were limitations in the pilot test, namely the small sample size (n=10) and lack of randomization or a control group. In addition, there was the potential for social desirability bias in responses to the palliative care attitudes measure because the outcomes were interview-administered. However, any individual-level variation in the assessment would have been accounted for in the pre-test measure given the within-subjects design. These limitations are reasonable given the in-depth intervention development process and initial evidence of efficacy.
Other limitations of this research included generalizability and scope. The intervention design and initial outcomes were derived from English-speaking adults with cancer and other stakeholders in the southern U.S. The investigators anticipate that locally-tailored interventions will be most impactful. For example, this intervention was designed for a healthcare system where palliative care is underutilized. In other systems where palliative care programs are already in high demand, other interventions may be more beneficial first, such as those focused on expanding palliative care program capacity by making the financial case that expansion will yield an appropriate rate of return on capital invested. Acknowledging that there are multiple appropriate models for palliative care delivery (37), the video intervention described in this study could also be used to increase the likelihood of conversations about primary palliative care with patients’ oncologists. Last, the investigation focused on intervention design processes, feasibility metrics, and the proximal outcomes of knowledge and attitudes. It was not designed to draw inferences about changes in palliative care utilization. A follow-up study tracking distal outcomes is ongoing.
Future Directions
Future investigations should examine whether psychoeducational interventions that improve palliative care knowledge and attitudes also result in increased utilization of early integrated palliative care and improved quality of life. The American Cancer Society has agreed to fund a four-year follow-up study, called the EMPOWER 3 RCT, which examines these longitudinal outcomes. Patients can watch the intervention on a tablet in the clinic or online at home. Since patients may have the opportunity to watch the video in less structured settings, we plan to examine additional process metrics related to video watching and playthrough rates. Administration of the three video versions will be tailored to patients’ level of health literacy, but it might be more practical in clinical care to let patients choose their preferred version after a brief description of the intervention. The RCT addresses oncologist-associated referral barriers by providing intervention patients with a question-prompt list to increase dialogue surrounding palliative care, regularly engaging the oncology team in palliative care education, and tracking study accrual in cancer center meetings to reinforce the importance of the research. This program of research will close the gap between the extensive evidence supporting early integrated palliative care (1-5) and historical low utilization rates (6-10).
In conclusion, we found that a psychoeducational intervention about palliative care was acceptable to patients, families, and clinicians and was feasible to implement clinically. The intervention was grounded in theory and guided by stakeholders. During a small pre-post pilot trial, preliminary evidence showed that patients with metastatic cancer experienced increased palliative care knowledge and attitudes after receiving the intervention. Although the study showed research using the intervention was feasible, decision interventions are not widely used in practice, so more research is needed to evaluate scalability. Moreover, future research will examine whether the intervention-associated improvements in knowledge and attitudes observed in this pilot study replicate in a larger RCT and drive increased utilization of early integrated palliative care.
Supplementary Material
Key Message.
This study describes the development and initial evaluation of a psychoeducational intervention to improve patients’ knowledge and attitudes about palliative cancer care. Results will help healthcare systems nationwide seeking to increase patient readiness to utilize palliative care and call for a follow-up randomized clinical trial testing the intervention’s efficacy.
Disclosures and Acknowledgements
Oliver Sartor has disclosed consultancies with the following: Advanced Accelerator Applications (AAA), Astellas, AstraZeneca, Bayer, Blue Earth Diagnostics, Inc., Bavarian Nordic, Bristol Myers Squibb, Clarity Pharmaceuticals, Clovis, Constellation, Dendreon, EMD Serono, Fusion, Isotopen Technologien Meunchen, Janssen, Myovant, Myriad, Noria Therapeutics, Inc., Novartis, Noxopharm, Progenics, POINT Biopharma, Pfizer, Sanofi, Tenebio, Telix, Theragnostics. Oliver Sartor has disclosed research grant support from the following: Advanced Accelerator Applications, AstraZeneca, Bayer, Constellation, Dendreon, Endocyte, Invitae, Janssen, Merck, Progenics, Sanofi, SOTIO.
This research was supported by National Institute of General Medical Sciences (U54 GM104940), the Louisiana Board of Regents (#LEQSF(2016-19)-RD-A-18), and the American Cancer Society (#134579-RSG-20-058-01-PCSM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kavalieratos D, Corbelli J, Zhang D, Dionne-Odom JN, Ernecoff NC, Hanmer J, et al. Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA. 2016;316(20):2104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaertner J, Siemens W, Meerpohl JJ, Antes G, Meffert C, Xander C, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: Systematic review and meta-analysis. BMJ. 2017;357:j2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoerger M, Wayser GR, Schwing G, Suzuki A, Perry LM. Impact of interdisciplinary outpatient specialty palliative care on survival and quality of life in adults with advanced cancer: A meta-analysis of randomized controlled trials. Annals of Behavioral Medicine. 2019;53(7):674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulton JJ, LeBlanc TW, Cutson TM, Porter Starr KN, Kamal A, Ramos K, et al. Integrated outpatient palliative care for patients with advanced cancer: A systematic review and meta-analysis. Palliative medicine. 2019;33(2):123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers JL, Perry LM, Hoerger M. Summarizing the evidence base for palliative oncology care: a critical evaluation of the meta-analyses. Clinical Medicine Insights: Oncology. 2020;14:1179554920915722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scibetta C, Kerr K, Mcguire J, Rabow MW. The costs of waiting: implications of the timing of palliative care consultation among a cohort of decedents at a comprehensive cancer center. Journal of Palliative Meicine. 2016;19(1):69–75. [DOI] [PubMed] [Google Scholar]
- 7.Blackhall LJ, Read P, Stukenborg G, Dillon P, Barclay J, Romano A, et al. CARE track for advanced cancer: Impact and timing of an outpatient palliative care clinic. Journal of Palliative Medicine. 2016;19(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roeland EJ, Triplett DP, Matsuno RK, Boero IJ, Hwang L, Yeung HN, et al. Patterns of palliative care consultation among elderly patients with cancer. Journal of the National Comprehensive Cancer Network. 2016;14(4):439–45. [DOI] [PubMed] [Google Scholar]
- 9.Bailey FA, Williams BR, Woodby LL, Goode PS, Redden DT, Houston TK, et al. Intervention to improve care at life’s end in inpatient settings: The BEACON trial. Journal of General Internal Medicine. 2014;29(6):836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Herder-van der Eerden M, van Wijngaarden J, Payne S, Preston N, Linge-Dahl L, Radbruch L, et al. Integrated palliative care is about professional networking rather than standardisation of care: a qualitative study with healthcare professionals in 19 integrated palliative care initiatives in five European countries. Palliative Medicine. 2018;32(6):1091–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: A systematic review. Journal of Pain and Symptom Management. 2007;34(1):94–104. [DOI] [PubMed] [Google Scholar]
- 12.Verkissen MN, Hjermstad MJ, Van Belle S, Kaasa S, Deliens L, Pardon K. Quality of life and symptom intensity over time in people with cancer receiving palliative care: Results from the international European Palliative Care Cancer Symptom study. PloS One. 2019;14(10):e0222988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen PB, Andrykowski MA. Tertiary prevention in cancer care: Understanding and addressing the psychological dimensions of cancer during the active treatment period. American Psychologist. 2015;70(2):134–45. [DOI] [PubMed] [Google Scholar]
- 14.Caruso R, Breitbart W. Mental health care in oncology. Contemporary perspective on the psychosocial burden of cancer and evidence-based interventions. Epidemiology and Psychiatric Sciences. 2020;29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaasa S, Loge JH, Aapro M, Albreht T, Anderson R, Bruera E, et al. Integration of oncology and palliative care: A Lancet Oncology Commission. The Lancet Oncology. 2018;19(11):e588–e653. [DOI] [PubMed] [Google Scholar]
- 16.Temel JS, Greer JA, El-Jawahri A, Pirl WF, Park ER, Jackson VA, et al. Effects of early integrated palliative care in patients with lung and GI cancer: A randomized clinical trial. Journal of Clinical Oncology. 2017;35(8):834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Temel JS, Sloan J, Zemla T, Greer JA, Jackson VA, El-Jawahri A, et al. Multisite, randomized trial of early integrated palliative and oncology care in patients with advanced lung and gastrointestinal cancer: Alliance A221303. Journal of Palliative Medicine. 2020;23(7):922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakitas M, Allen Watts K, Malone E, Dionne-Odom JN, McCammon S, Taylor R, et al. Forging a new frontier: Providing palliative care to people with cancer in rural and remote areas. Journal of Clinical Oncology. 2020;38(9):963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui D, Mori M, Watanabe SM, Caraceni A, Strasser F, Saarto T, et al. Referral criteria for outpatient specialty palliative cancer care: An international consensus. The Lancet Oncology. 2016;17(12):e552–e9. [DOI] [PubMed] [Google Scholar]
- 20.Jordan K, Aapro M, Kaasa S, Ripamonti C, Scotté F, Strasser F, et al. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Annals of Oncology. 2018;29(1):36–43. [DOI] [PubMed] [Google Scholar]
- 21.Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. Journal of Clinical Oncology. 2017;35(1):96–112. [DOI] [PubMed] [Google Scholar]
- 22.Hoerger M, Perry LM, Gramling R, Epstein RM, Duberstein PR. Does educating patients about the Early Palliative Care Study increase preferences for outpatient palliative cancer care? Findings from Project EMPOWER. Health Psychology. 2017;36(6):538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel P, Lyons L. Examining the knowledge, awareness, and perceptions of palliative care in the general public over time: A scoping literature review. American Journal of Hospice and Palliative Medicine®. 2019:481–7. [DOI] [PubMed] [Google Scholar]
- 24.Taber JM, Ellis EM, Reblin M, Ellington L, Ferrer RA. Knowledge of and beliefs about palliative care in a nationally-representative US sample. PloS One. 2019;14(8):e0219074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen MJ, Wellman JD. Evidence of palliative care stigma: The role of negative stereotypes in preventing willingness to use palliative care. Palliative & supportive care. 2019;17(4):374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry LM, Hoerger M, Malhotra S, Gerhart JI, Mohile S, Duberstein PR. Development and validation of the Palliative Care Attitudes Scale (PCAS-9): A measure of patient attitudes toward palliative care. Journal of Pain and Symptom Management. 2020;59(2):293–301. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann C, Swami N, Krzyzanowska M, Leighl N, Rydall A, Rodin G, et al. Perceptions of palliative care among patients with advanced cancer and their caregivers. Cmaj. 2016;188(10):E217–E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins A, McLachlan S-A, Philip J. Initial perceptions of palliative care: An exploratory qualitative study of patients with advanced cancer and their family caregivers. Palliative Medicine. 2017;31(9):825–32. [DOI] [PubMed] [Google Scholar]
- 29.Collins A, McLachlan S-A, Philip J. Communication about palliative care: A phenomenological study exploring patient views and responses to its discussion. Palliative Medicine. 2018;32(1):133–42. [DOI] [PubMed] [Google Scholar]
- 30.Stacey D, Samant R, Bennett C. Decision making in oncology: A review of patient decision aids to support patient participation. CA: A Cancer Journal for Clinicians. 2008;58(5):293–304. [DOI] [PubMed] [Google Scholar]
- 31.Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Viera A, Crotty K, et al. Health literacy interventions and outcomes: An updated systematic review. Evidence Report/Technology Assessment. 2011;199(1):1–941. [PMC free article] [PubMed] [Google Scholar]
- 32.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. New England Journal of Medicine. 2010;363(8):733–42. [DOI] [PubMed] [Google Scholar]
- 33.Kozlov E, Reid MC, Carpenter BD. Improving patient knowledge of palliative care: A randomized controlled intervention study. Patient Education and Counseling. 2017;100(5):1007–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graul A, Haggerty A, Stickley C, Kumar P, Morales K, Bogner H, et al. Effect of patient education on palliative care knowledge and acceptability of outpatient palliative care services among gynecologic oncology patients: A randomized controlled trial. Gynecologic Oncology. 2020;156(2):482–7. [DOI] [PubMed] [Google Scholar]
- 35.Kamal AH, Wolf S, Nicolla JM, Friedman F, Xuan M, Bennett AV, et al. Usability of PCforMe in patients with advanced cancer referred to outpatient palliative care: Results of a randomized, active-controlled pilot trial. Journal of Pain and Symptom Management. 2019;58(3):382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Firth AM, O’Brien SM, Guo P, Seymour J, Richardson H, Bridges C, et al. Establishing key criteria to define and compare models of specialist palliative care: A mixed-methods study using qualitative interviews and Delphi survey. Palliative Medicine. 2019;33(8):1114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hui D, Bruera E. Models of palliative care delivery for patients with cancer. Journal of Clinical Oncology. 2020;38(9):852–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoerger M, Perry LM, Korotkin BD, Walsh LE, Kazan AS, Rogers JL, et al. Statewide differences in personality associated with geographic disparities in access to palliative care: Findings on openness. Journal of Palliative Medicine. 2019;22(6):628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Institute P-COR. Prioritizing Comparative Effectiveness Research Questions for Patient-Centered Palliative Care Delivery for Adult Patients with Advanced Illnesses and Their Caregivers: A Stakeholder Workshop. . Arlington, VA; 2016. [Google Scholar]
- 40.Volk RJ, Llewellyn-Thomas H, Stacey D, Elwyn G. Ten years of the International Patient Decision Aid Standards Collaboration: Evolution of the core dimensions for assessing the quality of patient decision aids. BMC Medical Informatics and Decision Making 2013;13(S1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabin B, Glasgow RE. An implementation science perspective on psychological science and cancer: What is known and opportunities for research, policy, and practice. American Psychologist. 2015;70(2):211–20. [DOI] [PubMed] [Google Scholar]
- 42.Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, van der Weijden T. A systematic development process for patient decision aids. BMC Medical Informatics and Decision Making. 2013;13(2):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deverka PA, Lavallee DC, Desai PJ, Esmail LC, Ramsey SD, Veenstra DL, et al. Stakeholder participation in comparative effectiveness research: Defining a framework for effective engagement. Journal of comparative effectiveness research. 2012;1(2):181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elwyn G, O'connor AM, Bennett C, Newcombe RG, Politi M, Durand M-A, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi). PloS one. 2009;4(3):e4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozlov E, Carpenter BD, Rodebaugh TL. Development and validation of the Palliative Care knowledge scale (PaCKS). Palliative & Supportive Care. 2017;15(5):524–34. [DOI] [PubMed] [Google Scholar]
- 46.Cohen J Statistical power analysis for the behavioral sciences: Academic press; 2013. [Google Scholar]
- 47.American Society of Clinical Oncology. ASCO answers: Palliative care, improving quality of life for people with cancer and their families 2017. [Available from: (https://www.cancer.net/sites/cancer.net/files/palliative_care.pdf).
- 48.Center to Advance Palliative Care (CAPC). Palliative care: What you should know 2019. [Available from: (https://getpalliativecare.org/handouts-for-patients-and-families/).
- 49.Forsythe LP, Ellis LE, Edmundson L, Sabharwal R, Rein A, Konopka K, et al. Patient and stakeholder engagement in the PCORI pilot projects: Description and lessons learned. Journal of General Internal Medicine. 2016;31(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoerger M, Greer JA, Jackson VA, Park ER, Pirl WF, El-Jawahri A, et al. Defining the elements of early palliative care that are associated with patient-reported outcomes and the delivery of end-of-life care. Journal of Clinical Oncology. 2018;36(11):1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lustria MLA, Noar SM, Cortese J, Van Stee SK, Glueckauf RL, Lee J. A meta-analysis of web-delivered tailored health behavior change interventions. Journal of Health Communication. 2013;18(9):1039–69. [DOI] [PubMed] [Google Scholar]
- 52.Krebs P, Prochaska JO, Rossi JS. A meta-analysis of computer-tailored interventions for health behavior change. Preventive Medicine. 2010;51(3-4):214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison LG, Yardley L, Powell J, Michie S. What design features are used in effective e-health interventions? A review using techniques from critical interpretive synthesis. Telemedicine and e-Health. 2012;18(2):137–44. [DOI] [PubMed] [Google Scholar]
- 54.Tabibian B, Upadhyay U, De A, Zarezade A, Schölkopf B, Gomez-Rodriguez M. Enhancing human learning via spaced repetition optimization. Proceedings of the National Academy of Sciences. 2019;116(10):3988–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eisend M Two-sided advertising: A meta-analysis. International Journal of Research in Marketing. 2006;23(2):187–98. [Google Scholar]
- 56.Banas JA, Rains SA. A meta-analysis of research on inoculation theory. Communication Monographs. 2010;77(3):281–311. [Google Scholar]
- 57.Oncology Nursing Society. Position statement on palliative care. Oncology nursing forum; 2015. [PubMed] [Google Scholar]
- 58.Dutta MJ. Communicating about culture and health: Theorizing culture-centered and cultural sensitivity approaches. Communication Theory. 2007;17(3):304–28. [Google Scholar]
- 59.Torre JB, Lieberman MD. Putting feelings into words: Affect labeling as implicit emotion regulation. Emotion Review. 2018;10(2):116–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.