Fig. 1.
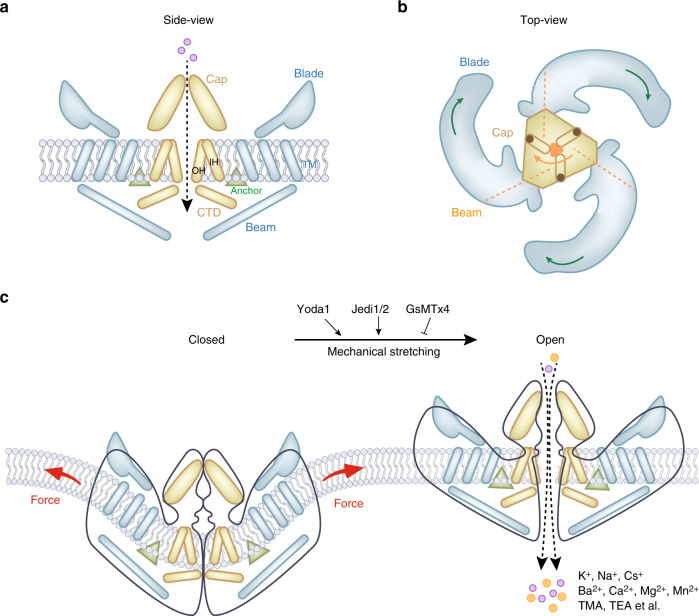
Mouse Piezo1 protein has a three-bladed, propeller-shaped homotrimeric architecture. a Side view of mouse Piezo1 channel. Piezo1 consists of a central ion-conducting pore modulus (yellow components) and the peripheral mechanotransduction modulus (blue component). The pore module contains the extracellular Cap structure, the transmembrane pore formed from three pairs of TMs, and the intracellular C-terminal domain (CTD). The peripheral mechanotransduction modulus includes a long beam-like structure, a peripheral blade, and a unique anchor domain. The anchor domain formed from a hairpin structure is connected to the CTD plane by the inner helix (IH) and outer helix (OH) pair, which maintains the integrity of the channel. The long beam structure supports and bridges the blade into the central pore module. b Top view of mouse Piezo1 channel. The large extracellular blade domains can curve the plasma membrane, and the three blades are assembled into functional trimers. c Mammalian Piezo1 proteins can be directly gated by membrane stretching, which is conserved throughout evolution. Yoda1 and Jedi1/2 are chemical activators of Piezo channels, and GsMTx4 is an antagonist of the Piezo1 channel. Piezo channels are nonselective cationic mechanosensitive channels that are permeable to alkali ions (K+, Na+, and Cs+), divalent cations (Ba2+, Ca2+, Mg2+, and Mn2+), and several organic cations (tetramethyl ammonium (TMA), tetraethyl ammonium (TEA)).56 Illustrations were modified from Wang et al.52 and Jiang et al.11