Figure 4.
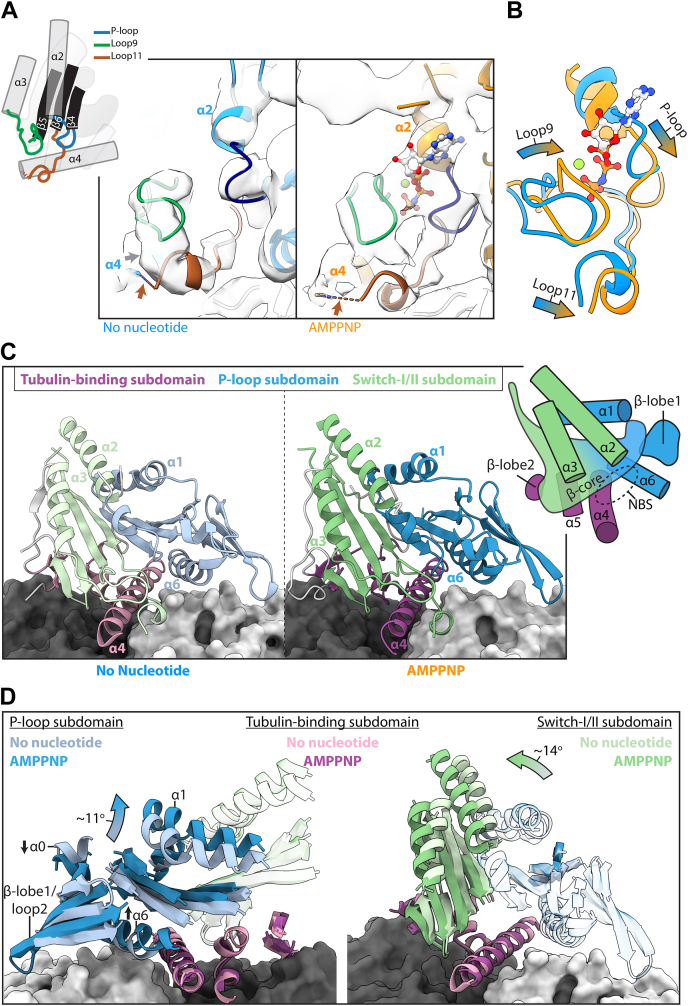
AMPNPP binding causes PfK5ΔL6-MD subdomain rearrangement and switch loop closure.A, rearrangements of PfK5ΔL6-MD switch loops upon AMPPNP binding, with a schematic showing connectivity of the switch loops to motor domain secondary structure elements on the left. Models and corresponding density are displayed on the right, with nucleotide-binding loops colored according to the schematic. The brown arrow indicates where there is missing density for loop11, and the gray arrow indicates connecting density between loop9 and 11. B, comparison of PfK5ΔL6-MD no nucleotide and AMPPNP nucleotide binding loops, demonstrating AMPPNP induced conformational changes. C, the PfK5ΔL6-MD no nucleotide state model, coloured according to kinesin subdomain, with α and β-tubulin depicted in light and dark gray surface rendering, respectively. D, PfK5ΔL6-MD subdomain rearrangement, showing no overall movement in the tubulin-binding subdomain, with rotations in the P-loop subdomain shown on the left, and the switch-I/II subdomain on the right.