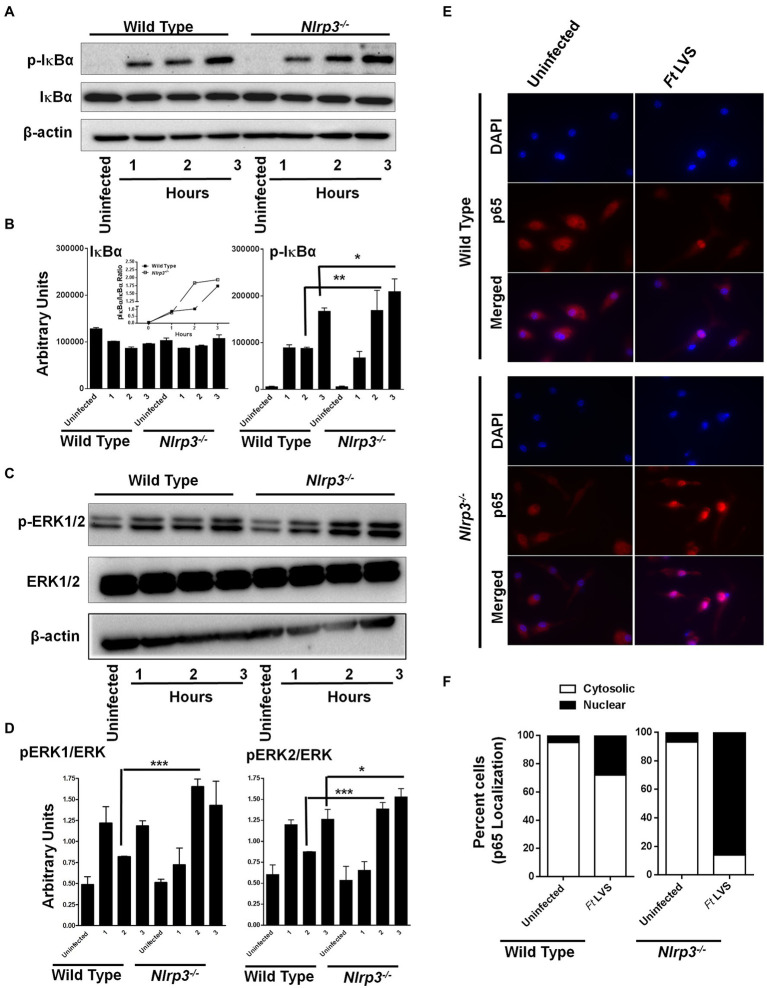
Figure 2.
Loss of Nlrp3 results in enhanced activation of NF-κβ and MAPK signaling. The wild-type and the Nlrp3−/− macrophages were infected with 100 MOI of F. tularensis (Ft) LVS for the indicated times, lysed and separated by SDS-PAGE, and immunoblotted with phosphorylated (p) and total IκBα- (A) and ERK1/2 (C) antibodies. β-actin was used as a loading control. Quantitation of IκBα, p-IκBα (B), ERK, and p-ERK bands (D) (n=2 blots). Uninfected macrophages were used as controls. The inset in B shows pIκBα/IκBα ratios. The data were analyzed by one-way ANOVA, and a p-value of 0.05 or less was considered significant. *p<0.05; **p<0.01; ***p<0.001. (E) Immunofluorescence staining was performed to detect cellular localization of the p65 subunit of NF-κβ in the wild type or the Nlrp3−/− macrophages infected with 100 MOI of Ft LVS (magnification 63×, red, p65; blue, nucleus). (F) Quantification of subcellular localization of p65 subunit of NF-κβ. At least 100 cells were counted manually in randomly selected fields. The results are expressed as percent macrophages showing cytosolic or the nuclear p65 localization. The data shown are representative of two independent experiments, each conducted with three biological replicates.