FIGURE 2.
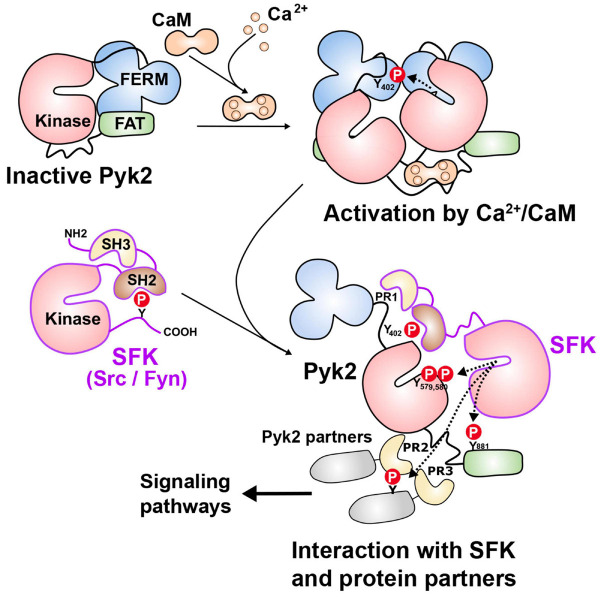
Pyk2 mechanisms of activation. In the absence of stimulus, Pyk2 is thought to be in a closed conformation due to possible intramolecular interactions between domains (top left). Activation can result from the binding of Ca2+/calmodulin (Ca2+/CaM) for which several potential sites have been identified, including in the FERM domain, the kinase domain, and the kinase-FAT linker (as shown here for simplicity). The binding of Ca2+/calmodulin is thought to facilitate dimerization or clustering of Pyk2 allowing intermolecular autophosphorylation of Tyr402 (Y402). Clustering can also result from interaction with partners including PSD-95, which can themselves dimerize or cluster in response to Ca2+/calmodulin (not shown). Autophosphorylation of Y402 provides a binding site for the SH2 domain of Src family kinases (SFKs especially Src and Fyn). Through their SH3 domain, SFKs can also bind to the proline-rich motif (PR1) located close to Y402. SFKs can then phosphorylate several tyrosine residues in Pyk2 including Y579 and Y580 in its activation loop, increasing its catalytic activity, and Y881 in the FAT domain, which can recruit additional partners with SH2 domains. SFKs, and possibly Pyk2 can phosphorylate associated proteins, including those interacting with PR2 and PR3 motifs through their SH3 domains. This can result in the formation of multiprotein complexes and the activation of various signaling pathways. Alternatively, activated SFKs can bypass the Ca2+/calmodulin-dependent step by directly phosphorylating Y402. In either case SFK and Pyk2 interactions provides a positive feedforward activation loop. See text for references.