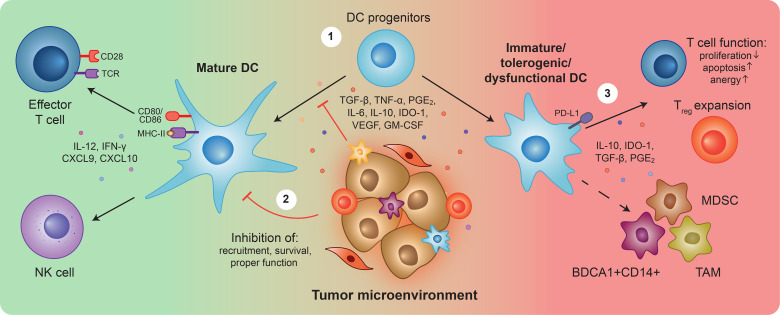
Figure 1.
Overview of Dendritic cell (dys)functions in cancer. Upon detection of tumor antigens and danger signals, dendritic cells (DCs) become activated, upregulate co-stimulatory surface molecules and secrete pro-inflammatory cytokines. Mature DCs can (cross)-present antigens, trigger tumor-specific T cell responses, and stimulate natural killer (NK) cell activity to unleash cytotoxic anti-tumor immunity (left). During tumor development and progression, the release of tumor-derived suppressive factors prevents DC progenitors from properly differentiating ①, and differentiated DCs from fulfilling their functions ②. Resulting immature, tolerogenic and/or dysfunctional DCs, characterized by the expression of TGF-β, IL-10, IDO-1, PGE2, and PD-L1, can inhibit T cell anti-tumor responses ③. Furthermore, they can differentiate into and favor the expansion of immunosuppressive populations such as myeloid-derived suppressor cells (MDSCs), BDCA1+CD14+ cells, and tumor-associated macrophages (TAMs). Overall, the impairment of DCs is a crucial step for tumor immune evasion, triggering a cascade of immunosuppression that hampers anti-tumor immunity and creates a propitious environment for tumor growth and metastasis initiation.