Abstract
The mitochondrial unfolded protein response (mitoUPR) is an evolutionarily conserved pathway that restores homeostasis to the mitochondria after various disturbances. This pathway has roles in both resistance to exogenous stressors and longevity. The mitoUPR is mediated by the transcription factor ATFS-1/ATF-5, which modulates the expression of genes involved in protein folding, metabolism and stress resistance. MitoUPR activation in C. elegans is most commonly evaluated through transcriptional reporter strains for the mitochondrial chaperones HSP-6 and HSP-60. In order to obtain a more comprehensive view of transcriptional changes resulting from activation of the mitoUPR, we compared gene expression changes from three different mitoUPR-activating interventions: mutation of nuo-6, RNA interference (RNAi) knockdown of spg-7,and constitutive activation of ATFS-1. We specifically focused on gene expression changes that are dependent on ATFS-1. From this comparison, we identified 61 high confidence target genes that can be used to monitor mitoUPR activation. Notably, neither hsp-6 nor hsp-60 were significantly upregulated under all three mitoUPR activating conditions. We ranked the 61 genes according to the magnitude of upregulation and identify multiple genes that may serve as robust readouts of mitoUPR activation.
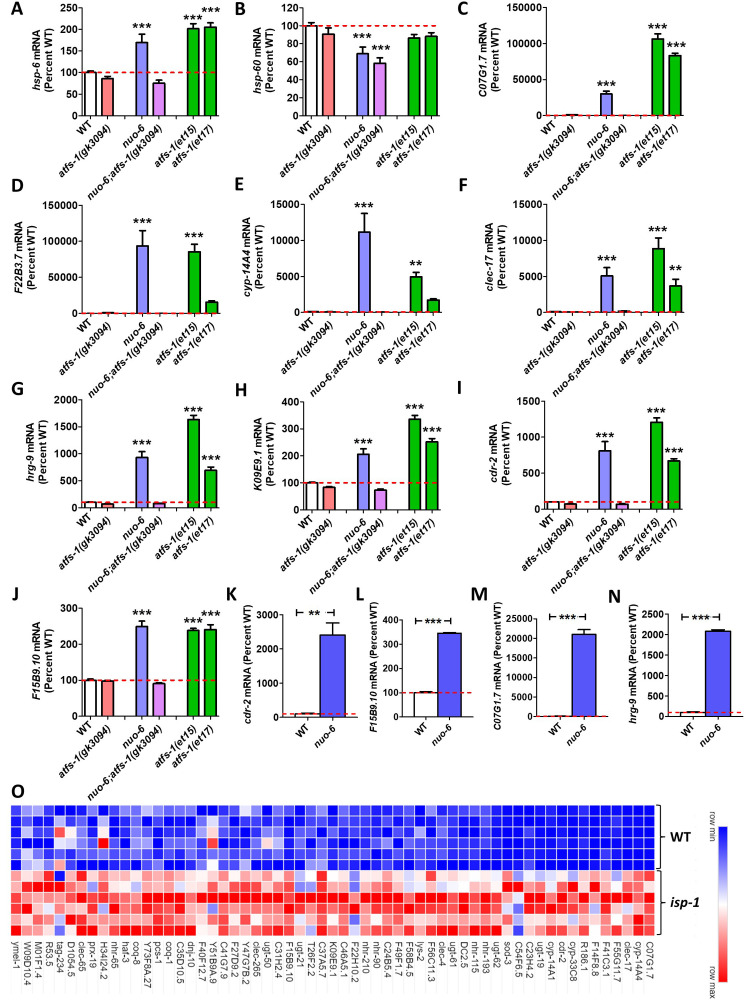
Figure 1. Levels of gene expression under conditions of mitoUPR activation.
To compare the efficacy of using different target genes to monitor activation of the mitoUPR, we examined the expression of each gene under conditions of ATFS-1 activation. To activate ATFS-1, we used a nuo-6 mutation (blue bars) or the constitutively active atfs-1(et15) and atfs-1(et17) mutations (green bars). ATFS-1 activation was prevented using an atfs-1 deletion mutation gk3094 in wild-type and nuo-6 worms (red bars and purple bars, respectively). Expression is shown as a percentage of wild type worms (white bars). A. There is a significant increase in hsp-6 mRNA under conditions of ATFS-1 activation that is prevented by disruption of atfs-1. B. The levels of hsp-60 are not increased in nuo-6 mutants or either constitutively active atfs-1 mutants. C-F.The mitoUPR target genes C01G1.7, F22B3.7, cyp-14A4 and clec-17 exhibit a marked increased in expression under conditions of ATFS-1 activation with a magnitude much greater than hsp-6. G-J. The mitoUPR target genes hrg-9, K09E9.1, cdr-2 and F15B9.10 show a large increase in expression relative to their standard deviation under conditions of ATFS-1 activation. K-N. The expression of mitoUPR target genes cdr-2, F15B9.10, C07G1.7 and hrg-9 show increased expression in nuo-6 worms by quantitative RT-PCR. O. Heat map comparing the expression of identified mitoUPR target genes between wild-type and long-lived mitochondrial mutant isp-1 worms. All but 4 of the 61 genes are significantly upregulated in isp-1 mutants. Expression levels in panels A-J were determined from our previously published RNA sequencing data (Wu et al., 2018). Expression represents counts per million from six biological replicates that has been normalized to wild-type. Heat map was generated using our previously published RNA sequencing data (Senchuk et al., 2018). Error bars indicates SEM. **p<0.01, ***p<0.001. Statistical significance was assessed using a one-way ANOVA with Dunnett’s multiple comparison test, except in panels K-N where a student’s t-test was used.
Description
The mitochondrial unfolded protein response (mitoUPR) is a stress response pathway that promotes cell survival and restores mitochondrial function when mitochondrial health is compromised (Haynes et al. 2013; Jovaisaite et al. 2014; Shpilka and Haynes 2018). While a mitoUPR was first reported in mammalian cells (Zhao et al. 2002), the initial work on the mitoUPR in C. elegans was performed by Yoneda et al. who found that treatment with ethidium bromide, which affects the replication and expression of mitochondrial DNA, increased the expression of the mitochondrial chaperone gene hsp-6 (Yoneda et al. 2004). Based on this observation, they generated hsp-6p::gfp and hsp-60p::gfp reporter strains to further study the mitoUPR. They found that either RNA interference (RNAi) targeting spg-7, the worm homolog of paraplegin, or RNAi targeting other genes encoding mitochondrial proteins resulted in activation of the hsp-6p::gfp and hsp-60p::gfp reporter strains (Yoneda et al. 2004).
The hsp-6p::gfp and hsp-60p::gfp reporter strains were used to identify other components of the mitoUPR including the transcriptional regulator UBL-5, the transcription factor DVE-1, the protease ClpP, the protein import channel HAF-1, and the transcription factor ATFS-1 (Benedetti et al. 2006; Haynes et al. 2007; Haynes et al. 2010; Nargund et al. 2012). Under normal conditions, the mitoUPR transcription factor, ATFS-1, is imported into the mitochondria through the HAF-1 import channel and degraded by the protease ClpP. However, when mitochondria are impaired, ATFS-1 import into the mitochondria is blocked. Instead, the nuclear localization signal (NLS) of ATFS-1 drives ATFS-1 into the nucleus. In the nucleus, ATFS-1 acts with DVE-1 and UBL-5 to upregulate genes that restore mitochondrial homeostasis, including genes involved in mitochondrial protein folding and metabolism. In addition to responding to disruptions in mitochondrial function and integrity, the mitoUPR also plays important roles in resistance to stress (Pellegrino et al. 2014; Campos et al. 2021; Soo et al. 2021) and longevity (Durieux et al. 2011; Houtkooper et al. 2013; Bennett et al. 2014; Wu et al. 2018).
Previous studies from our laboratory and others have used RNA sequencing (RNA-seq) or microarrays to examine gene expression under conditions that induce ATFS-1 activation. Nargund et al. identified 366 genes that are upregulated by spg-7 RNAi in an ATFS-1-dependent manner (Nargund et al. 2012). We previously identified 1704 genes that are upregulated in nuo-6 mutants in an ATFS-1-dependent manner (Senchuk et al. 2018; Wu et al. 2018) and 529 genes that are upregulated in both atfs-1(et15) and atfs-1(et17) constitutively active atfs-1 mutants (Rauthan et al. 2013; Wu et al. 2018).
In order to establish a list of mitoUPR target genes that are consistently upregulated across all three ATFS-1-activating conditions, we examined the overlap between these datasets. We identified a total of 61 genes that are upregulated in nuo-6 worms in an ATFS-1-dependent manner, upregulated by spg-7 RNAi in an ATFS-1-dependent manner, and upregulated in both atfs-1(et15) and atfs-1(et17) constitutively active mutants (Extended data, Column A,B). Surprisingly, neither hsp-6 nor hsp-60 were among the 61 genes on this list. In fact, hsp-60 was not identified in any of the three datasets. It is uncertain why spg-7 RNAi increases fluorescence in the hsp-60p::GFP reporter strain (Yoneda et al. 2004) but hsp-60 was not identified as one of the genes upregulated by spg-7 RNAi (Nargund et al. 2012). One possibility may be that hsp-60 expression is primarily upregulated during development in response to spg-7 RNAi and other mitochondrial insults. Then the half-life of the HSP-60::GFP protein might allow the increase in HSP-60::GFP to still be observed at adulthood even after the hsp-60 mRNA has returned to baseline.
In order to rank the different mitoUPR target genes, each gene was scored by adding together their expression levels in nuo-6, atfs-1(et15) and atfs-1(et17) mutants and subtracting their expression level in nuo-6;atfs-1 mutants. By this metric, 42 of the 61 mitoUPR target genes exhibited a higher score than hsp-6 (Extended data, Column J). The top genes of C07G1.7, F22B3.7, cyp-14A4, and clec-17 had scores ranging from 17084-21882, compared to a score of 350 for hsp-6. The expression of these genes compared to hsp-6 and hsp-60 is shown in Fig 1A-F.
In order to control for variability, we divided this score by the average standard deviation from WT, atfs-1(gk3094), nuo-6, nuo-6;atfs-1(gk3094), afts-1(et15) and atfs-1(et17). By this metric, 27 of the 61 mitoUPR target genes showed a higher score than hsp-6 (Extended data, Column K). The top genes of C07G1.7, hrg-9, K09E9.1, cdr-2 and F15B9.10 had variability-corrected scores of 26-38 compared to 15 for hsp-6. The expression of these genes is shown in Fig 1G-J.
To determine if these mitoUPR target genes are being activated directly by binding of ATFS-1, we examined the data from a previous chromatin immunoprecipitation sequencing (ChIP-seq) experiment that identified genes bound by ATFS-1 after treatment with spg-7 RNAi (Nargund et al. 2015). We found that 22 of the 61 mitoUPR target genes exhibited binding of ATFS-1 after spg-7 RNAi (Extended data, Column L). This suggests that the expression of these genes is directly modulated by ATFS-1, while the expression of the other mitoUPR target genes may be regulated indirectly, or perhaps bound by ATFS-1 under different conditions than were utilized in the ChIP-seq study.
Our results suggest that the genes identified here may be better for monitoring the activation of the mitoUPR by quantitative RT-PCR or RNA-seq than hsp-6 and hsp-60. To that end, we have designed and validated primers for quantitative RT-PCR to measure the levels of two of the highest-ranked direct targets, cdr-2 and F15B9.10, and two of the highest ranked indirect targets, C07G1.7 and hrg-9. We confirmed that all four sets of primers can efficiently measure activation of the mitoUPR in nuo-6 worms compared to wild-type worms (Fig 1K-N).
Finally, to validate this list of 61 genes for monitoring the activation of the mitoUPR, we examined the long-lived mitochondrial mutant isp-1, which we have previously shown to have increased activation of a hsp-6p:::gfp reporter strain and significantly overlapping gene expression changes with constitutively active atfs-1 mutants (Wu et al. 2018). 57 of the 61 genes were found to be significantly upregulated in isp-1 mutants (Extended data, Fig 1O).
Overall, this work has identified a gene expression signature that can be used to monitor the activation of the mitoUPR in RNA-seq, microarray or qPCR studies. These genes can be used to complement the use of hsp-6p::gfp and hsp-60p::gfp reporter strains, and may provide a more robust measure of mitoUPR activation in studies measuring mRNA.
Methods
Strains. Worms were grown on NGM plates seeded with OP50 bacteria at 20°C. We previously performed RNA sequencing on wild-type(N2), atfs-1(gk3094), nuo-6(qm200), nuo-6(qm200);atfs-1(gk3094), atfs-1(et15), atfs-1(et17) and isp-1(qm150) worms (Senchuk et al. 2018; Wu et al. 2018). This RNA sequencing data was re-analyzed to generate Figure 1, panels A-J and Figure 1, panel O. The quantitative RT-PCR results in Figure 1, panels K-N were generated for this publication using wild- type and nuo-6(qm200) worms
Quantitative RT-PCR. Worms from an overnight limited lay were allowed to grow to the prefertile young adult stage. Worms were washed three times with M9 buffer and frozen in Trizol. RNA was isolated by phenol-chloroform extraction using Trizol reagent as previously described (Machiela et al. 2016). RNA was converted to cDNA using a High Capacity cDNA Reverse Transcription Kit (Life Technologies) according to the manufacturer’s protocol. qPCR was performed in a Viia 7 RT-PCR machine from Applied Biosystems real-time thermal cycler using PowerUp SYBR Green Master Mix kit (Applied Biosystems). RNA was collected from three biological replicates and normalized to the levels of act-3. Primer sequences:
cdr-2 (L: CGAGCCTCATTTGGAAAGAA, R: GCATCTGCCGCTGTAACTTT)
F15B9.10 (L: CCGGACAGTTTCAAGAATGC, R: CACTGAGGATCCAATGTCCA)
hrg-9 (L: TGGAATATTGAGTGGCGTTG, R: CCTCCTCTACTTGGTGCATGT)
C07G1.7 (L: GCTGAAGAAGCTTCAACCGTAG, R: TCTCGTGTCAATTCCGGTCT)
Analysis of gene expression data. Lists of differentially expressed genes were obtained from (Nargund et al. 2012;Wu et al. 2018). The raw data from these experiments is available on NCBI GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE38196, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE110984). We used BioVenn (https://www.biovenn.nl/index.php) to generate lists of overlapping genes between gene sets. Genes upregulated in nuo-6 worms in an ATFS-1-dependent manner are genes that are upregulated in nuo-6 mutants but not nuo-6;atfs-1 mutants. To rank mitoUPR target genes, expression of each gene was normalized to wild-type. The score for each gene was determined by summing the normalized expression in nuo-6, atfs-1(et15) and atfs-1(et17) mutants and subtracting three times the expression in nuo-6;atfs-1 mutants. To generate a variability-corrected score, this score was divided by the average standard deviation for each of the strains. Binding of ATFS-1 was determined from a previous chromatin immunoprecipitation (ChIP) study (Nargund et al. 2015). Data from this study is available on NCBI GEO: (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE63803).
Reagents
| Strain | Genotype | Source |
| N2 | wild-type | Wild isolate from Bristol |
| MQ1333 | nuo-6(qm200) | Yang, Hekimi. 2010. |
| VC3201 | atfs-1(gk3094) | C. elegans Reverse Genetics Core Facility at the University of British Columbia |
| QC115 | atfs-1(et15) | Rauthan et al. 2013. |
| QC117 | atfs-1(et17) | Rauthan et al. 2013. |
| MQ887 | isp-1(qm150) | Feng et al. 2001. |
| JVR477 | nuo-6(qm200); atfs-1(gk3094) | Wu et al. 2018. |
Acknowledgments
Acknowledgments
We would like to thank Dr. Paige Rudich for reviewing this manuscript and providing suggestions for improvement. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P30 OD010440). We would also like to acknowledge the C. elegans knockout consortium and the National Bioresource Project of Japan for providing strains used in this research.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR; http://www.cihr-irsc.gc.ca/; JVR) and the Natural Sciences and Engineering Research Council of Canada (NSERC; https://www.nserc-crsng.gc.ca/index_eng.asp; JVR) and the National Institute of General Medical Sciences (NIGMS; https://www.nigms.nih.gov/; JVR) by grant number R01 GM121756. JVR received a Senior Research Scholar career award from the Fonds de Recherche du Quebec Santé (FRQS) and Parkinson Quebec. SKS received a scholarship from FRQS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics. 2006 Jul 01;174(1):229–239. doi: 10.1534/genetics.106.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CF, Vander Wende H, Simko M, Klum S, Barfield S, Choi H, Pineda VV, Kaeberlein M. Activation of the mitochondrial unfolded protein response does not predict longevity in Caenorhabditis elegans. Nat Commun. 2014 Mar 24;5:3483–3483. doi: 10.1038/ncomms4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos JC, Wu Z, Rudich PD, Soo SK, Mistry M, Ferreira JC, Blackwell TK, Van Raamsdonk JM. Mild mitochondrial impairment enhances innate immunity and longevity through ATFS-1 and p38 signaling. EMBO Rep. 2021 Oct 01;:e52964–e52964. doi: 10.15252/embr.202152964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011 Jan 01;144(1):79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes CM, Fiorese CJ, Lin YF. Evaluating and responding to mitochondrial dysfunction: the mitochondrial unfolded-protein response and beyond. Trends Cell Biol. 2013 Mar 13;23(7):311–318. doi: 10.1016/j.tcb.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes CM, Yang Y, Blais SP, Neubert TA, Ron D. The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol Cell. 2010 Feb 26;37(4):529–540. doi: 10.1016/j.molcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell. 2007 Oct 01;13(4):467–480. doi: 10.1016/j.devcel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, Auwerx J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013 May 23;497(7450):451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovaisaite V, Mouchiroud L, Auwerx J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J Exp Biol. 2014 Jan 01;217(Pt 1):137–143. doi: 10.1242/jeb.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiela E, Dues DJ, Senchuk MM, Van Raamsdonk JM. Oxidative stress is increased in C. elegans models of Huntington's disease but does not contribute to polyglutamine toxicity phenotypes. Neurobiol Dis. 2016 Aug 18;96:1–11. doi: 10.1016/j.nbd.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Nargund AM, Fiorese CJ, Pellegrino MW, Deng P, Haynes CM. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt). Mol Cell. 2015 Mar 12;58(1):123–133. doi: 10.1016/j.molcel.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 2012 Jun 14;337(6094):587–590. doi: 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino MW, Nargund AM, Kirienko NV, Gillis R, Fiorese CJ, Haynes CM. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature. 2014 Sep 28;516(7531):414–417. doi: 10.1038/nature13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauthan M, Ranji P, Aguilera Pradenas N, Pitot C, Pilon M. The mitochondrial unfolded protein response activator ATFS-1 protects cells from inhibition of the mevalonate pathway. Proc Natl Acad Sci U S A. 2013 Mar 25;110(15):5981–5986. doi: 10.1073/pnas.1218778110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senchuk MM, Dues DJ, Schaar CE, Johnson BK, Madaj ZB, Bowman MJ, Winn ME, Van Raamsdonk JM. Activation of DAF-16/FOXO by reactive oxygen species contributes to longevity in long-lived mitochondrial mutants in Caenorhabditis elegans. PLoS Genet. 2018 Mar 01;14(3):e1007268–e1007268. doi: 10.1371/journal.pgen.1007268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpilka T, Haynes CM. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol. 2017 Nov 22;19(2):109–120. doi: 10.1038/nrm.2017.110. [DOI] [PubMed] [Google Scholar]
- Soo SK, Traa A, Rudich PD, Mistry M, Van Raamsdonk JM. Activation of mitochondrial unfolded protein response protects against multiple exogenous stressors. Life Sci Alliance. 2021 Sep 28;4(12) doi: 10.26508/lsa.202101182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Senchuk MM, Dues DJ, Johnson BK, Cooper JF, Lew L, Machiela E, Schaar CE, DeJonge H, Blackwell TK, Van Raamsdonk JM. Mitochondrial unfolded protein response transcription factor ATFS-1 promotes longevity in a long-lived mitochondrial mutant through activation of stress response pathways. BMC Biol. 2018 Dec 18;16(1):147–147. doi: 10.1186/s12915-018-0615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci. 2004 Jul 27;117(Pt 18):4055–4066. doi: 10.1242/jcs.01275. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002 Sep 01;21(17):4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]