Figure 1. Closed and open CFTR structures may not suffice to explain CFTR’s response to ATP following prolonged channel closure.
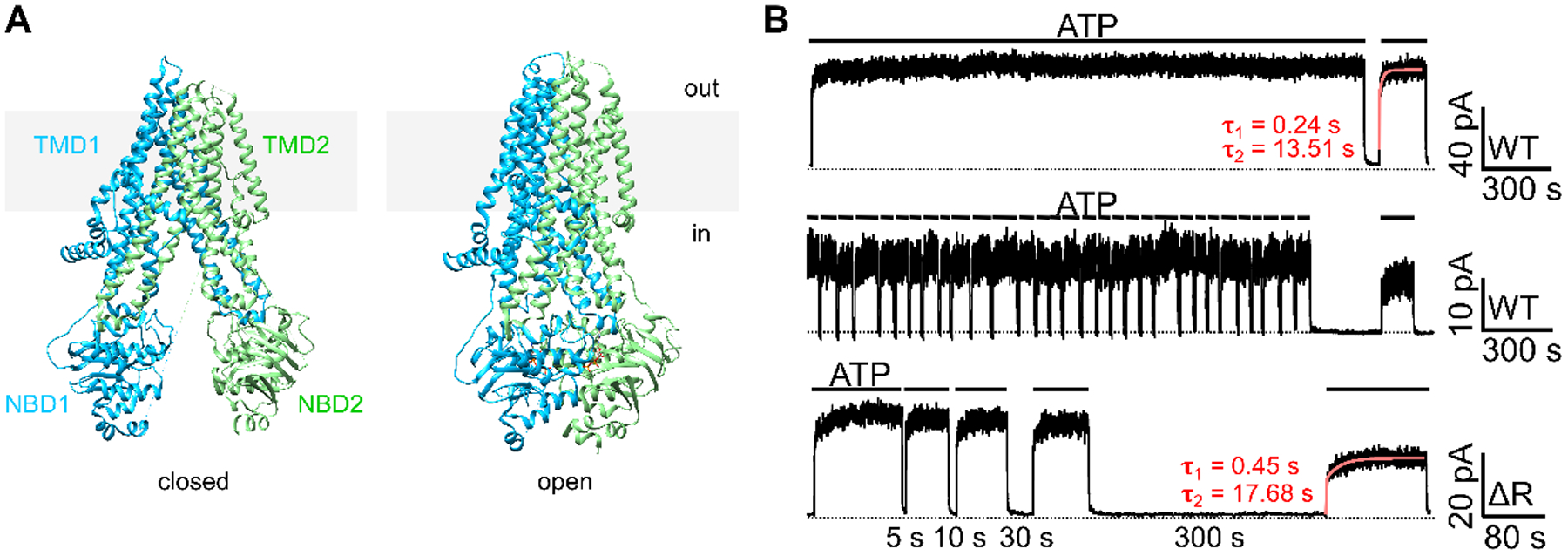
(A) CryoEM structures of the presumed closed (left) and open (right) channel conformations of CFTR. The closed conformation is represented by the structure of unphosphorylated, ATP-free CFTR (left; PDB: 5UAK; Liu et al., 2017); whereas the open conformation is represented by phosphorylated, ATP-bound CFTR (right; PDB: 6MSM; Zhang et al., 2018). Of note, although the pore of the ATP-bound CFTR structure is not wide enough for the passage of a dehydrated chloride, the overall architecture should nonetheless closely resemble an open conformation (Zhang et al., 2018). For clarity, the R domain (a.a. 637–845) was removed from the structures. Grey rectangles represent the lipid bilayer. In: intracellular side. Out: extracellular side. (B) Prolonged channel closure alters the response of WT- and ΔR-CFTR to ATP. Once activated by PKA and ATP, WT-CFTR currents remained stable in the continuous presence of ATP for 30 minutes (top trace). Repeated brief removal of ATP (10 seconds, 30 times) did not affect the current amplitude in the presence of ATP (middle trace), yet a 300-second prolonged withdrawal of ATP resulted in irreversible loss of the currents (~25% in this experiment). Like WT-CFTR, ΔR-CFTR, after prolonged depletion of ATP, failed to achieve the initial current amplitude prior to the 300-second removal of ATP (bottom trace). The red curves mark the double-exponential fit, yielding a fast (τ1) and a slow (τ2) time constants. Similar observations were made in 4 patches with WT-CFTR and 5 patches with ΔR-CFTR. Inward currents are displayed as upward deflections in all figures.
